The Arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. If we look at the equation that this Arrhenius equation calculator uses, we can try to understand how it works: k = A\cdot \text {e}^ {-\frac {E_ {\text {a}}} {R\cdot T}}, k = A ⋅ e−R⋅T Ea, where: The Math / Science The Arrhenius Equation, k = A⋅ e− Ea RT k = A ⋅ e - E a RT, can be rewritten (as shown below) to show the change from k 1 to k 2 when a temperature change from T 1 to T 2 takes place. The Activation Energy equation using the Arrhenius formula is: Ea = R⋅ ln(k2 k1) 1 T 1 − 1 T 2 E a = R ⋅ ln ( k 2 k 1) 1 T 1 - 1 T 2 where:

Arrhenius Calculations YouTube
Arrhenius equation calculator Natural Language Math Input Extended Keyboard Examples Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music… Arrhenius Equation Calculator - Free Online Calculator Learn how to use the Arrhenius equation calculator with a step-by-step procedure. Get the Arrhenius equation calculator available online for free only at BYJU'S. Login Study Materials NCERT Solutions NCERT Solutions For Class 12 NCERT Solutions For Class 12 Physics The Arrhenius equation, from a chemical point of view, is: k = A\cdot e^ {\frac {-E_ {\text {a}}} {R \cdot T}} k = A ⋅ e R⋅T −Ea We can identify many elements in the Arrhenius equation: k k is the rate constant, usually expressed in the units \text {M}^ {1-n}\text {s} M1−ns, where n n is the reaction order; The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the rate constant, and therefore rate, of a chemical reaction. At higher temperatures, the probability that two molecules will collide is higher.

16.2 The Arrhenius equation (HL) YouTube
You can find the activation energy for any reactant using the Arrhenius equation: E_\mathrm {a} = -R × T × \ln\biggl (\frac {k} {A}\biggr) E a = −R × T × ln(Ak) where: R R — Gas constant. It is equal to \mathrm {8.314\ J/ (K\!\cdot\!mol)} 8.314 J/(K⋅mol); T T — Temperature of the surroundings, expressed in Kelvins; k k — Reaction rate coefficient. Arrhenius Equation Calculator. K = Rate Constant; A = Frequency Factor; EA = Activation Energy; T = Temperature; R = Universal Gas Constant ; Temperature has a profound influence on the rate of a reaction. Arrhenius showed that the rate constant (velocity constant) of a reaction increases exponentially with an increase in temperature. The 'Arrhenius Equation Calculator' is a free online tool that calculates the rate constant in the Arrhenius equation for a reaction. In this calculator, you can enter the Activation Energy (Ea), Temperatur, Frequency factor and the rate constant will be calculated within a few seconds. How to Use Arrhenius Equation Calculator? The Arrhenius equation is a simple and accurate formula for the temperature dependence of the chemical reaction rate constant. The Arrhenius equation can be used to show the effect of a change of temperature on the rate constant and on the rate of the chemical reaction. k = A*e (-Ea/RT) A = pre-exponential or frequency factor.
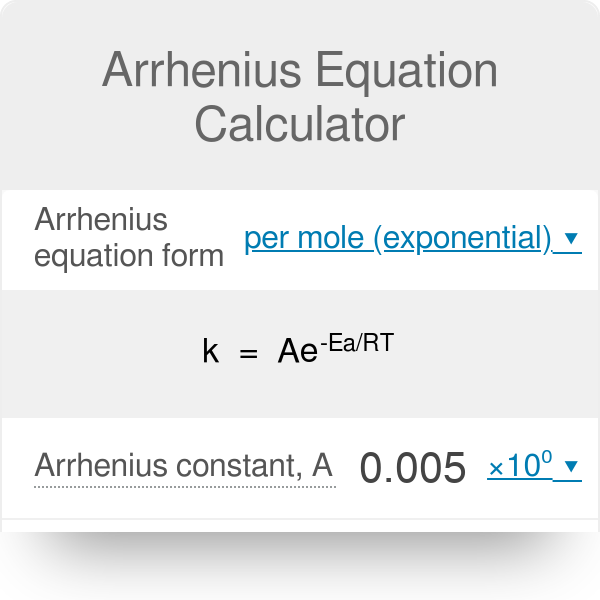
30+ arrhenius equation calculator MujahidTaylore
The Arrhenius equation is used to calculate the rate constant (k) of a chemical reaction as a function of temperature (T), activation energy (Ea), and the pre-exponential factor (A). The equation is expressed as: k = A * exp (-Ea / (R * T)) Where: k is the rate constant A is the pre-exponential factor or frequency factor Ea is the activation energy k = A × e - (Ea/RT) Where: k is the rate constant of the reaction, A is the pre-exponential factor, which is the rate constant at infinite temperature, E a is the activation energy of the reaction, R is the universal gas constant, and T is the absolute temperature in Kelvin. Real Life Application
The exponential term in the Arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. Because the rate of a reaction is directly proportional to the rate constant of a reaction, the rate increases exponentially as well.. Calculate the activation energy if the pre-exponential. This calculator calculates the effect of temperature on reaction rates using the Arrhenius equation. k=A*exp (-Ea/R*T) where k is the rate coefficient, A is a constant, E a is the activation energy, R is the universal gas constant, and T is the temperature (in degrees Kelvin). R has the value of 8.314 x 10 -3 kJ mol -1 K -1.
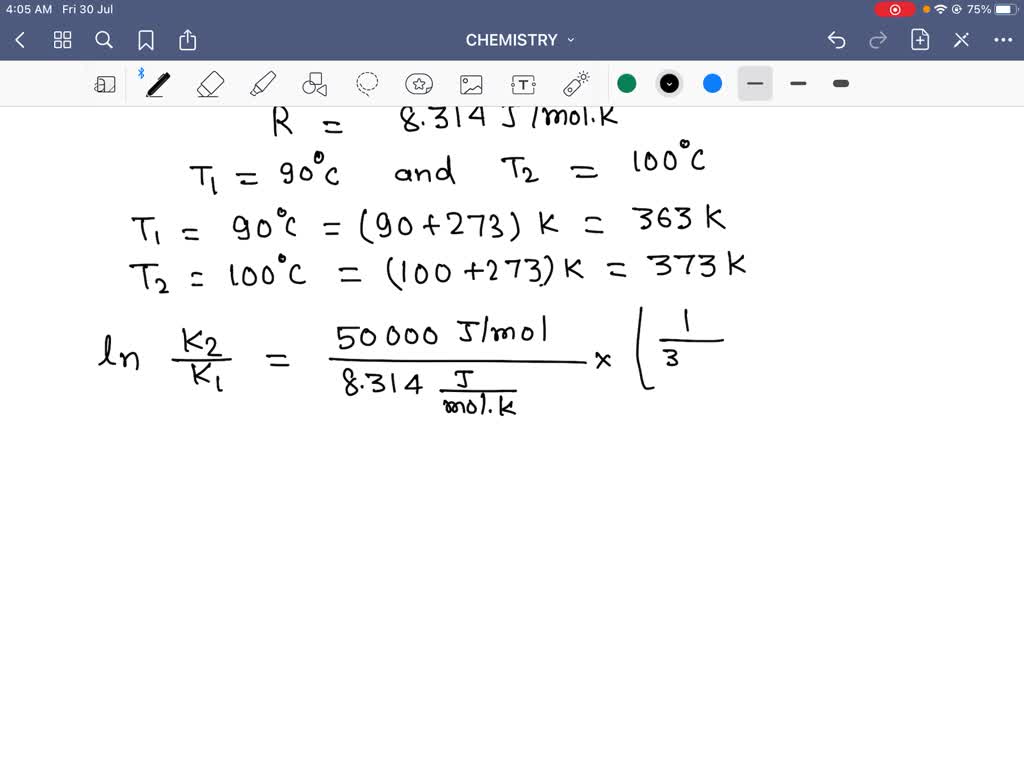
Apply the Arrhenius equation to calculate how many ti… SolvedLib
The Arrhenius Equation Calculator is used to compute the frequency factor of a chemical reaction. The user must know the rate constant, activation energy, and the temperature at which the reaction is taking place. The Arrhenius Equation comes from the collision theory of molecules. The Arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature as where k is the rate constant (frequency of collisions resulting in a reaction), T is the absolute temperature (in Kelvin or degree Rankine ), A is the pre-exponential factor or Arrhenius factor or frequency factor.




