Drawing the Bohr Model of Sulfur. Sulfur is the 16 th element of the Periodic table. It belongs to Period 3 and group 16. The information that we can infer from the above-mentioned Sulfur box is as follows: • The atomic number of Sulfur is 16. • The electronic configuration of Sulfur is [Ne] 3s 2 3p 4. In this video we'll look at the atomic structure and Bohr model for the Sulfur atom (S). We'll use a Bohr diagram to visually represent where the electrons a.
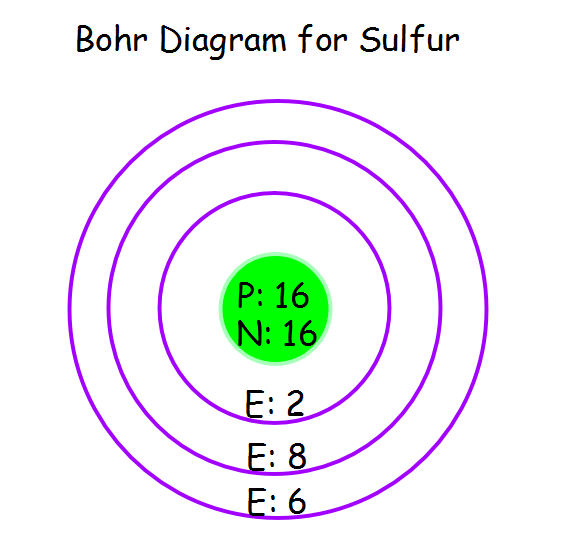
Bohr Diagram The Element Sulfur
The Bohr model of Sulfur (S) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 6 electrons. Sulfur is neutral and its atomic number is 16, hence, the number of protons and electrons available for its Bohr diagram is also 16. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is. Sulfur / Sulphur has 2 electrons in its first shell, 8 in its second, 6 in its third.Check me out: http://www.chemistnate.com The Bohr Rutherford diagram of sulfur illustrates that it has 16 electrons distributed in different energy levels or shells around the nucleus. The first energy level contains two electrons, and the second energy level contains eight electrons. The outermost or valence shell contains six electrons.

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
On the far left of Figure 3.6.1 3.6. 1 are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves. 6.2 The Bohr Model; 6.3 Development of Quantum Theory; 6.4. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as. phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their. Bohr model of calcium: (CC BY-SA 2.0 uk;Greg Robson): Answer b. Bohr model of sulfur: (CC BY-SA 2.0 uk; Greg Robson). Valence electrons are located in the highest energy level of an atom. When drawing a Bohr diagram, the valence electrons would be present in the outermost electronic level/shell (furthest away from the nucleus). Bohr's expression for the quantized energies is: En = − k n2, n = 1, 2, 3,.. E n = − k n 2, n = 1, 2, 3,.. In this expression, k is a constant comprising fundamental constants such as the electron mass and charge and Planck's constant. Inserting the expression for the orbit energies into the equation for Δ E gives.
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
Here's how you can draw the Bohr model of sulfur step by step. #1 Write protons, neutrons, and electrons of sulfur atom. #2 Draw nucleus of sulfur atom. #3 Draw 1 st electron shell. #4 Draw 2 nd electron shell. #5 Draw 3 rd electron shell. Let's break down each step in detail. The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three different energy levels, or orbits. Physics suggests that electrons do not physically exist as "points," but teachers use the Bohr atom model with fixed electrons as a way to simplify atomic structure. Creating the model requires the ability to cut with scissors and use glue.
Bohr model of Elements. 1. Hydrogen (H) 1. 2. Helium (He) 2. 3. Lithium (Li) That is, sulfur is an anion element. S + 2e - → S 2-. The electron configuration of sulfide ion (S 2-) is 1s 2 2s 2 2p 6 3s 2 3p 6. This electron configuration shows that sulfide ion (S 2-) has three shells and the 3rd shell has eight electrons.
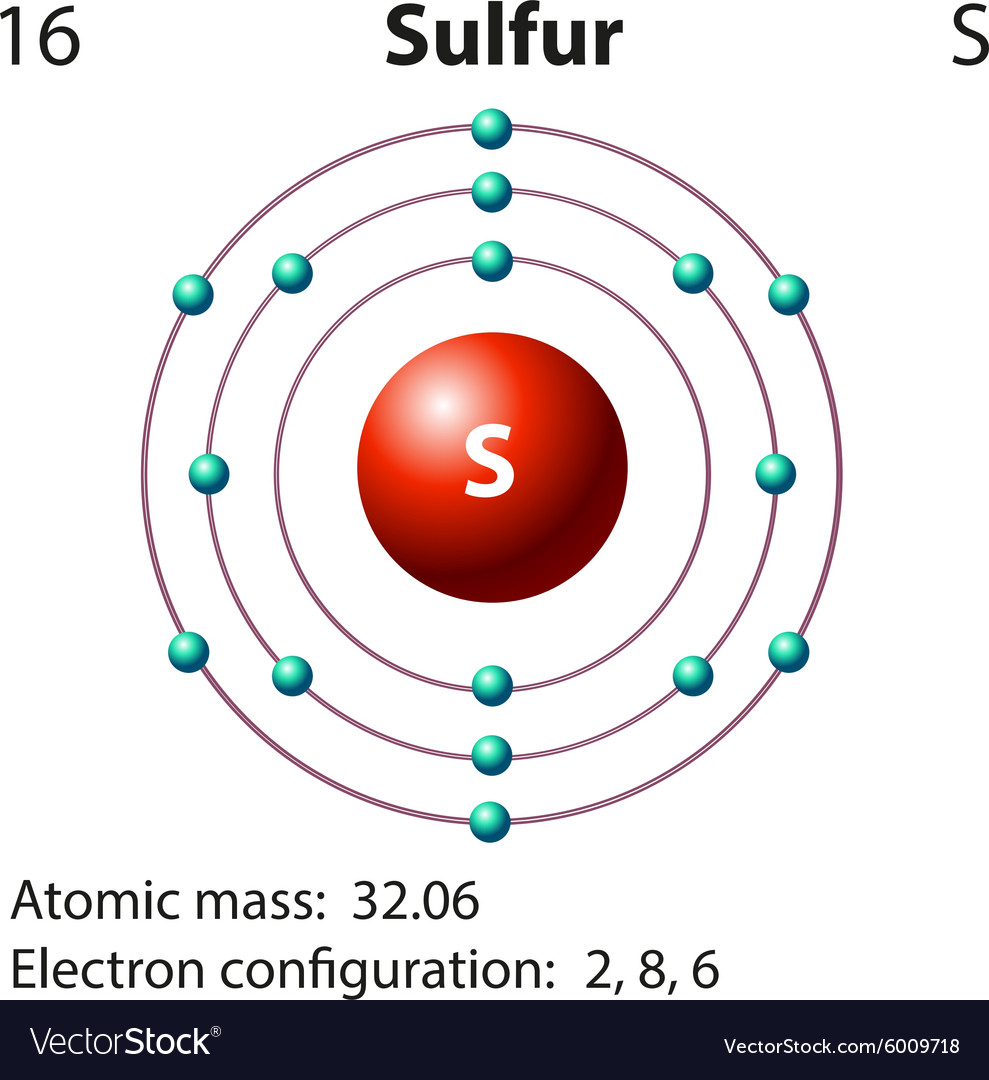
Diagram representation of the element sulfur Vector Image
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons What is the Bohr diagram for sulfur? Bohr Model: In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,.. The sphere n = 1 can accommodate two, the n=2-3 spheres eight, and the n = 4 sphere fourteen electrons. Answer and Explanation: 1.


:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)

