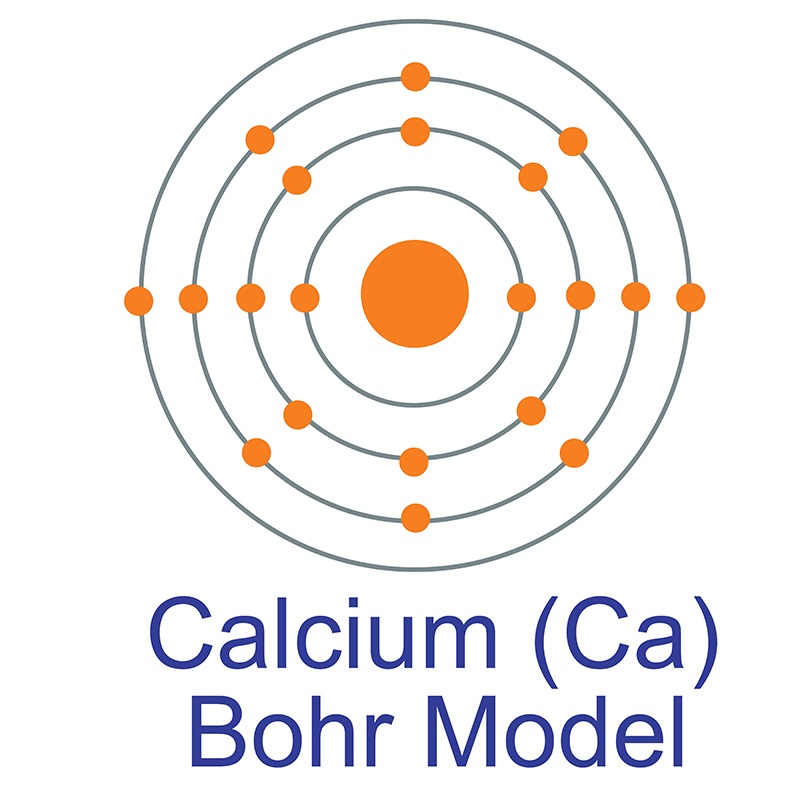
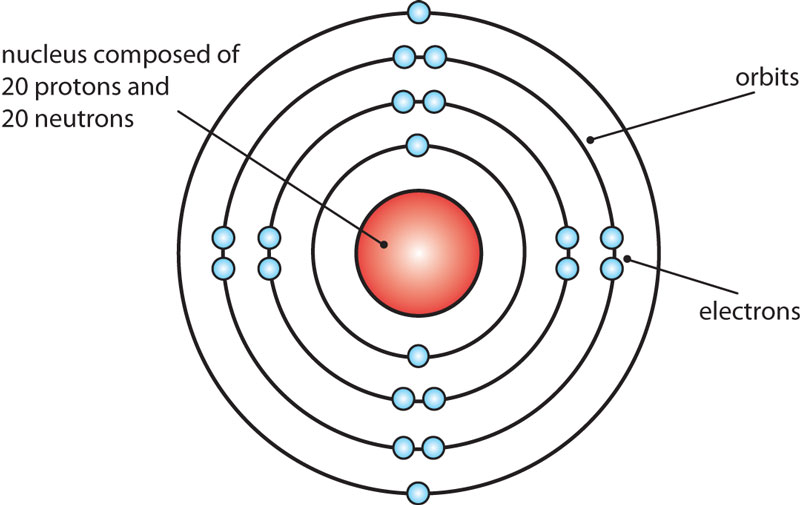
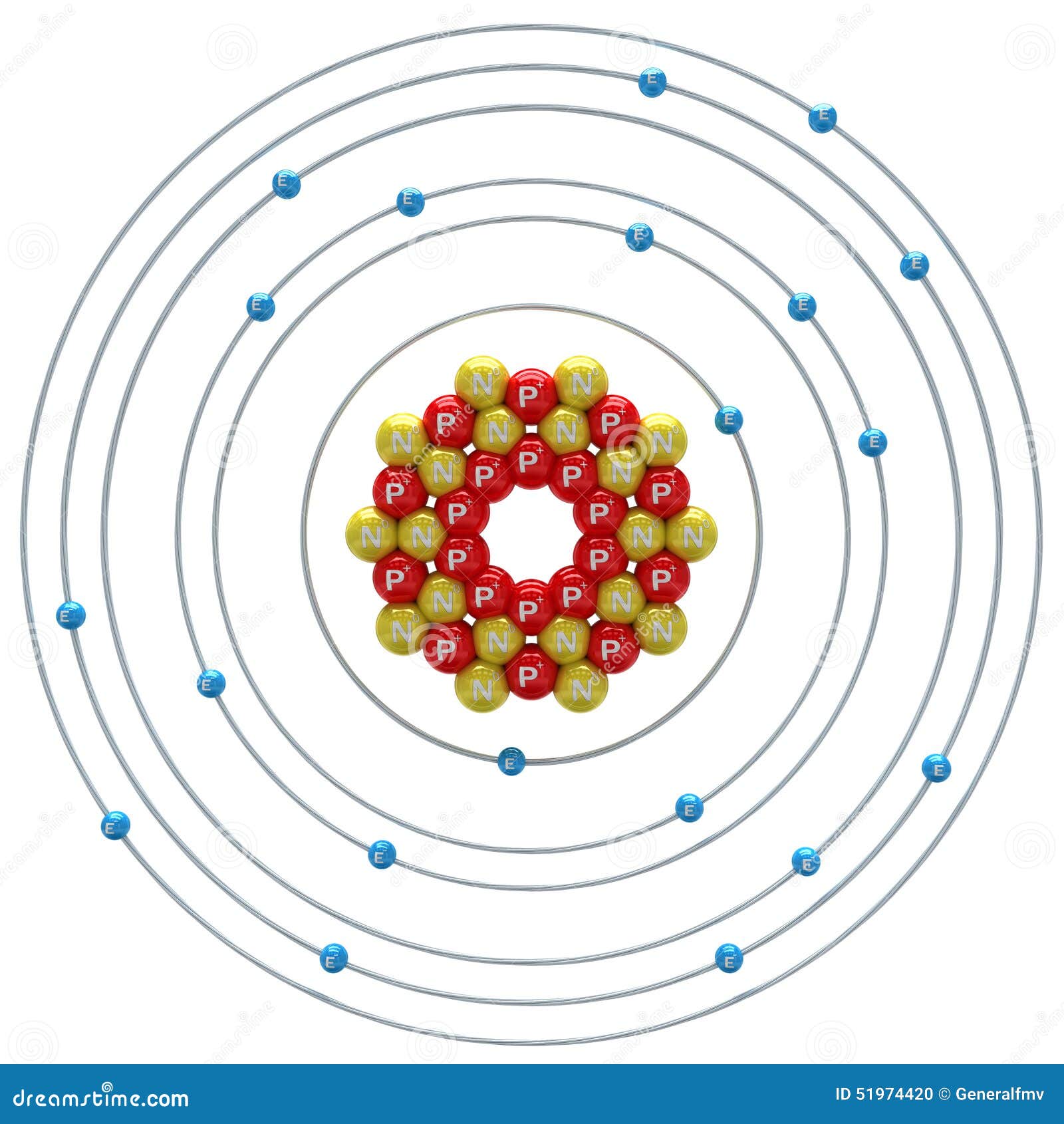
Atomic Structure (Bohr Model) for Calcium (Ca) Wayne Breslyn 722K subscribers Join Subscribe Subscribed 84 10K views 1 year ago In this video we'll look at the atomic structure and Bohr. Calcium Bohr model The Bohr model of calcium contains a nucleus having 20 protons and 20 neutrons in the center, and around this nucleus, there are four electron shells containing 20 electrons. Atomic Structure (Bohr Model) for Calcium (Ca) Watch on Contents Steps #1 Write protons, neutrons, and electrons of calcium atom
Calcium (Ca) AMERICAN ELEMENTS
The Bohr model of calcium is a simplified representation of the atom's nuclear structure, named after Danish physicist Niels Bohr. It depicts the nucleus as a small, positively charged ball with electrons orbiting around it in circular paths at fixed distances. Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is. In the Bohr model, there are a few rules that will help you draw accurate diagrams. Electrons must occupy the lowest available shell, closest to the nucleus. The maximum number of electrons that can fill each shell is: two in the first shell, eight in the second shell, eight in the third shell.

Bohr Model Representation Calcium Atom Number Stock Vector (Royalty
The Bohr model Google Classroom Learn how Bohr models are used to represent atoms. Atoms are way too small to see with the naked eye (and even most microscopes). So, we represent atoms using models. Models help us visualize atomic structure. They also help us explain and predict the behavior of atoms. Bohr model of calcium: (CC BY-SA 2.0 uk;Greg Robson): Answer b. Bohr model of sulfur: (CC BY-SA 2.0 uk; Greg Robson). Valence electrons are located in the highest energy level of an atom. When drawing a Bohr diagram, the valence electrons would be present in the outermost electronic level/shell (furthest away from the nucleus). An atom can have. The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to. 9.4: The Bohr Model - Atoms with Orbits is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. LICENSED UNDER. Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.
Calcium Bohr diagram Calcium
The Bohr model of the hydrogen atom ( Z = 1) or a hydrogen-like ion ( Z > 1 ), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy ( hν ). [1] Bohr model of the atom In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n. Electrons can jump from one orbit to another by emitting or absorbing energy.
Steps to draw the Bohr Model of Calcium atom 1. Find the number of protons, electrons, and neutrons in the Calcium Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom Course: Class 9 Chemistry (India) > Unit 4. Lesson 1: Models of an atom. Discovery of the electron and nucleus. Rutherford's gold foil experiment. Drawback of the Rutherford model. Bohr's model of an atom. Atomic structure. Science >. Class 9 Chemistry (India) >.
Calcium Atom on a White Background Stock Illustration Illustration of
The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. He postulated that the electron was restricted to certain orbits characterized by discrete energies. The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus.. Suggest a reason for the observation that the spectrum of calcium is more complicated than the spectrum of.




