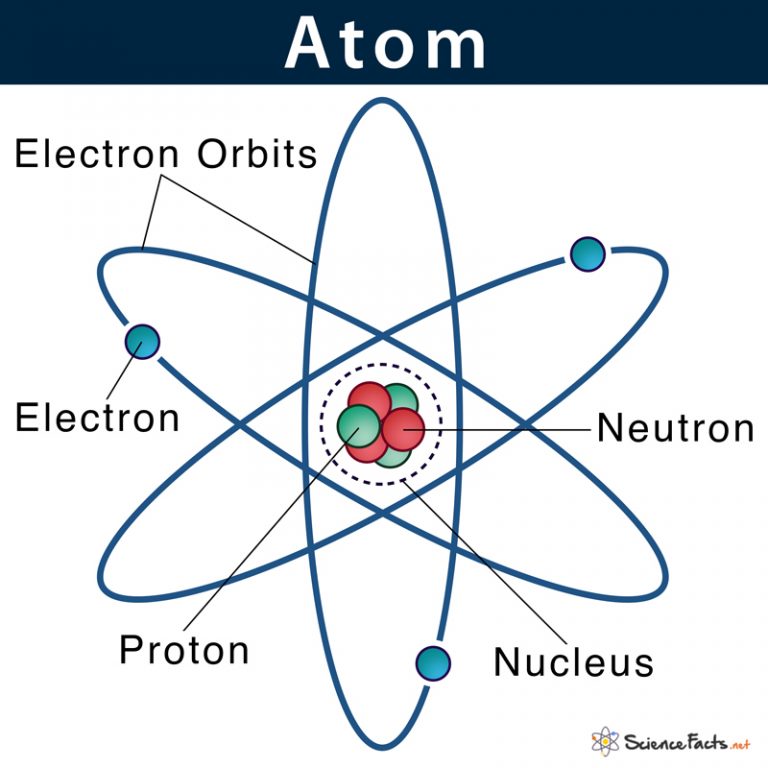
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. Atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral). The electrons are negatively charged particles that orbit the nucleus's centre. Democritus came up with the concept that matter is composed of atoms.
Atom Definition, Structure & Parts with Labeled Diagram
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10 −10 metre. The radius of an atom measures 1-2 Å. Physical Chemistry (Essentials) - Class 11 8 units · 52 skills. Unit 1 Welcome to physical chemistry. Unit 2 Structure of atom. Unit 3 Some basic Concepts of Chemistry. Unit 4 Redox reactions. Unit 5 Gaseous state. Unit 6 Thermodynamics. Unit 7 Chemical Equilibrium. Unit 8 Ionic equilibrium. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number . For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The following diagram summarizes the basic facts of the structure of the atom. ATOM NUCLEUS The nucleus is the center of mass (A), but does not significantly contribute to volume. It is made up of: PROTONS: Mass = 1 amu, charge = +1 NEUTRONS: Mass = 1 amu, charge = 0 ELECTRONS
Structure Of An Atom Class 9 Science Notes Leverage Edu
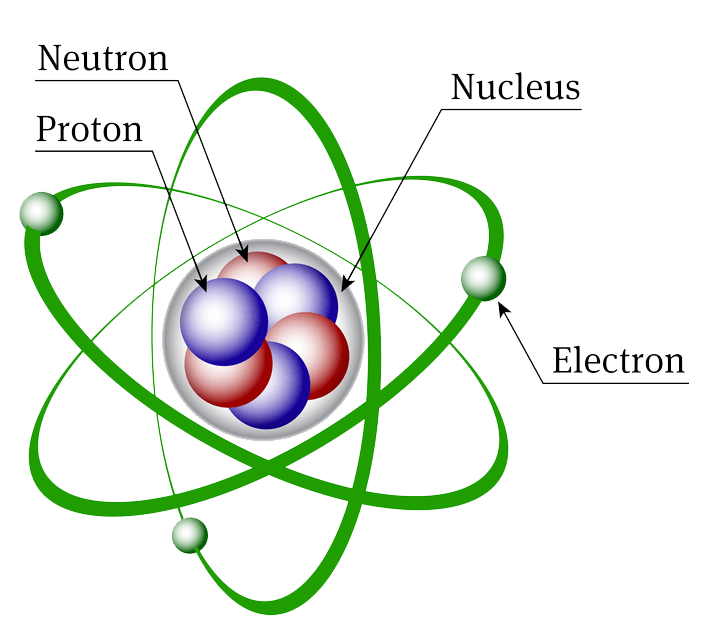
Atom The atom is the basic particle of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically-bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties.These properties determine an element's physical state at room temperature—gas, solid, or liquid. Figure 10.5a: Generalized energy-level diagram: generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale) (credit: Chemistry (OpenStax), CC BY 4.0 ). Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example. Figure 2.4. 1: The Geiger-Marsden Experimental Setup. Experiments using this setup were used to investigate the structure of atoms. In this experiment, most of the particles traveled straight through the foil, but some alpha particles were deflected off to one side. Some were even deflected back toward the source.
Atomic Structure (GCSE) — the science hive
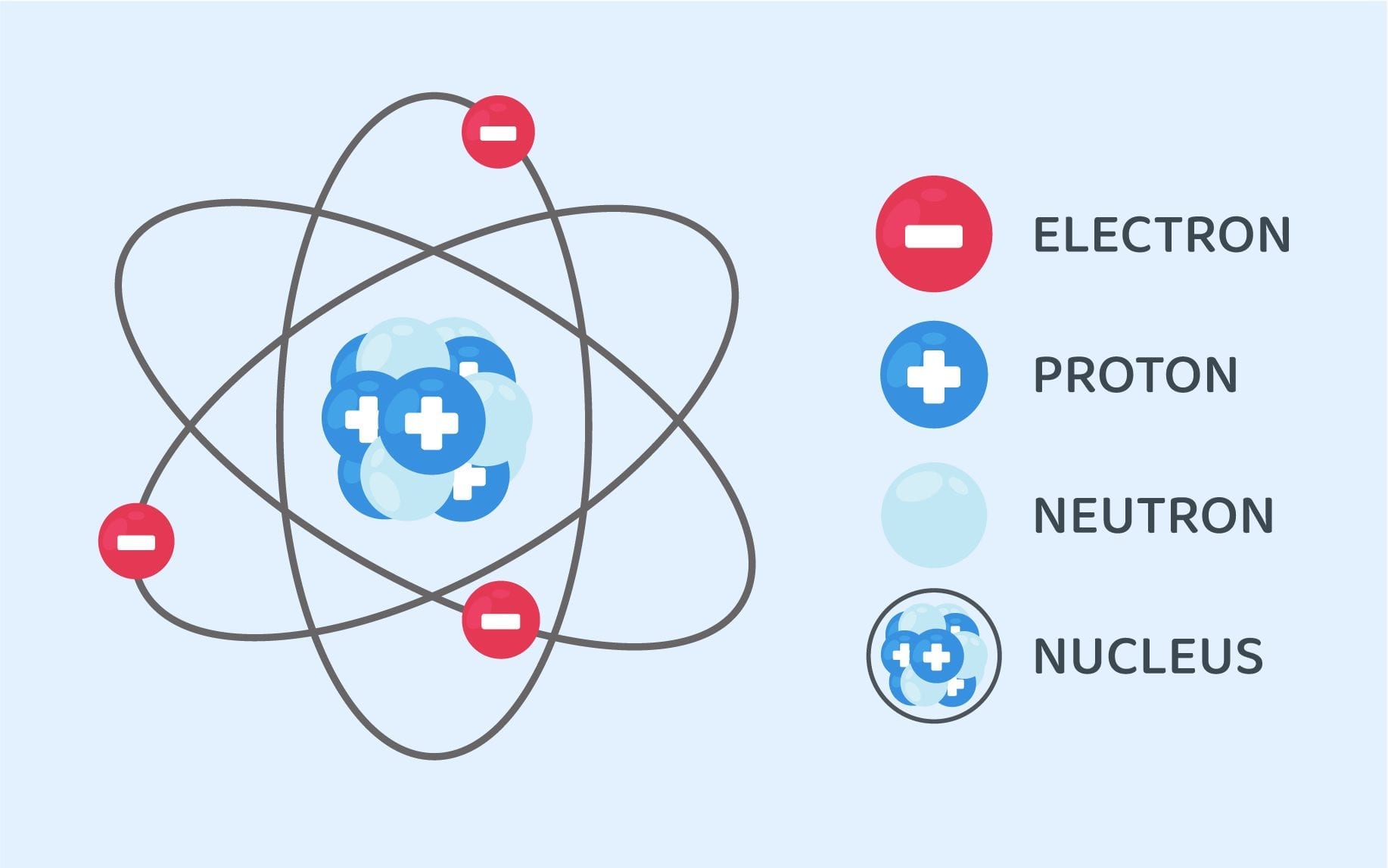
The atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus. Download Complete Chapter Notes of Structure of Atom Download Now The atomic structure of these building blocks is very interesting. The protons and neutrons are located in the center of the atom, while the electrons are quite far from the center. The number of protons in the nucleus of an atom determines its atomic number and its identity as a specific element. For example, all hydrogen atoms have one proton.
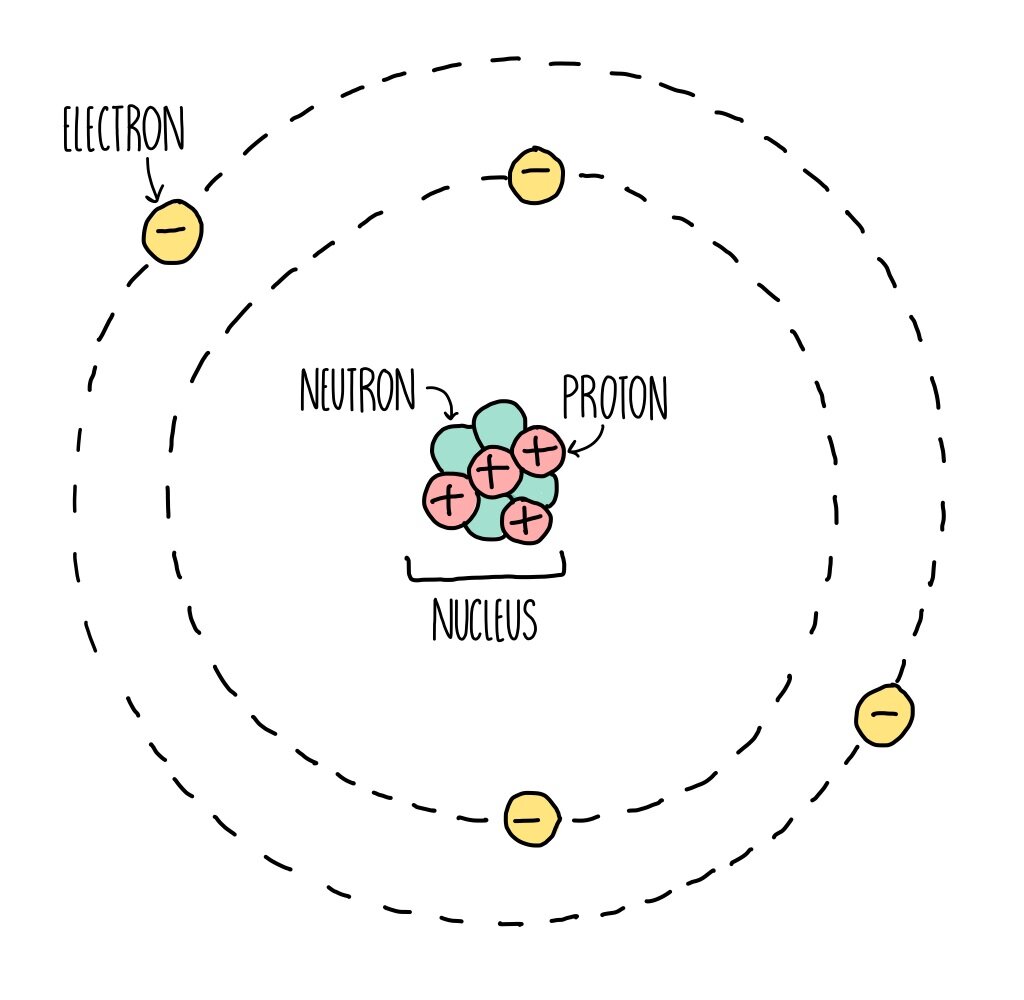
Basic Diagram of an Atom Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Map: Chemistry - The Central Science (Brown et al.) 2: Atoms, Molecules, and Ions
Atomic Structure Biochemistry
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). nucleus . This is surrounded by electrons arranged in shells. The nucleus is tiny compared to the atom as a whole: the radius of an atom is about 0.1 nm (1 × 10 -10 m) the radius of a nucleus (1 ×.




