A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc. I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle.
Lewis Dot Diagram For H2o Free Diagram For Student
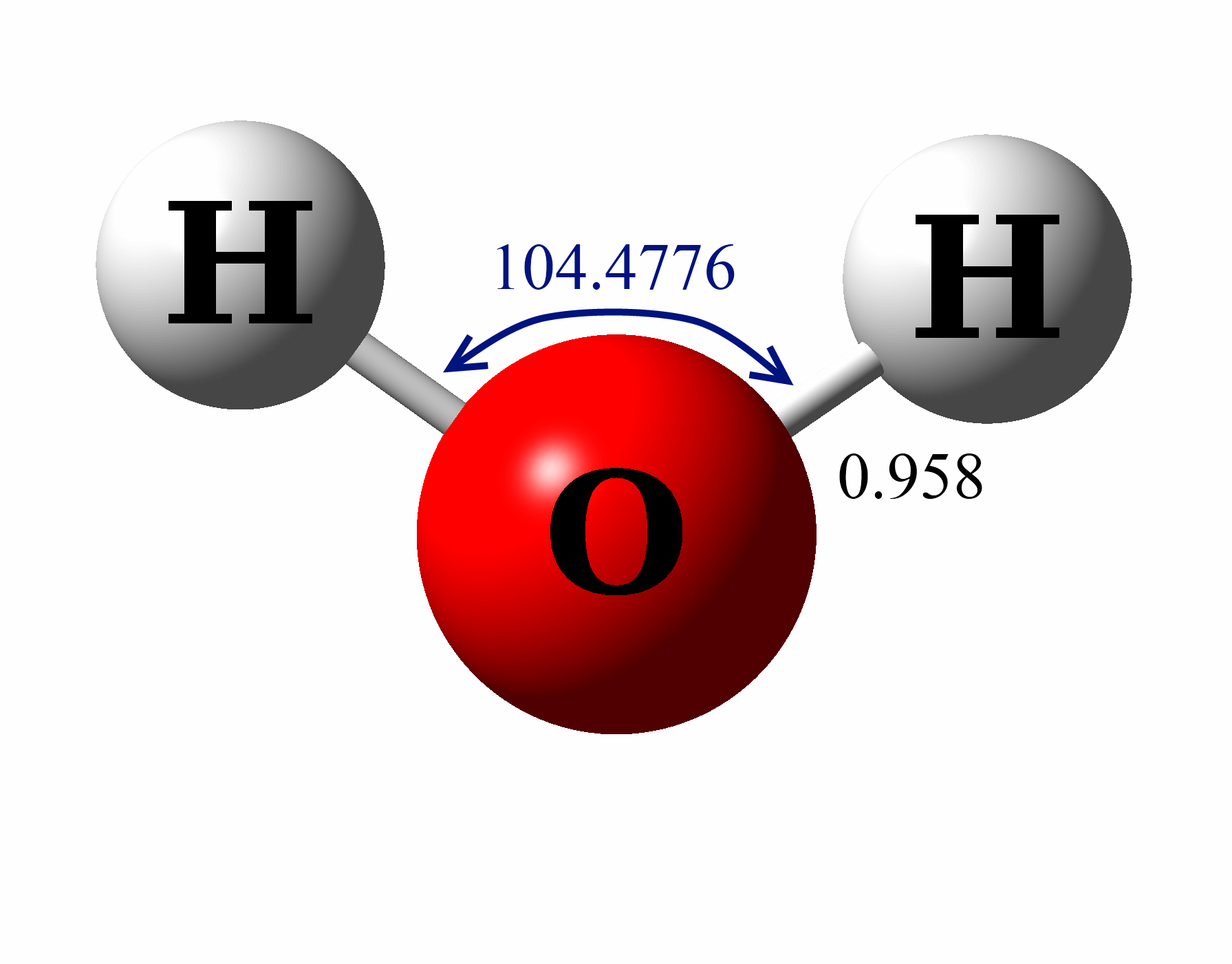
As a result, the water molecule's molecular geometry is angular or v-shaped. The bond angle in a water molecule (104.5°) Hybridization of H 2 O. The Lewis structure shows two single sigma bonds between the oxygen and hydrogen atoms. Besides that, these bonds leave the oxygen atom with two lone pairs of electrons. Using Lewis Dot Symbols to Describe Covalent Bonding.. We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed. Using Lewis Dot Symbols to Describe Covalent Bonding.. We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed. Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation
A video explanation of how to draw the Lewis Dot Structure for Water, along with information about the compound including Formal Charges, Polarity, Hybrid Or. The Lewis Dot Structure of Water H2O http://chemin10.com How to draw the bonds in H2O, and how the valence electrons of water contribute to its shape and pol. The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Moreover, these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule. It is the reason why the bond angle that should have. Water is a polar molecules. Water forms four hydrogen bonds, 1 for each hydrogen and 2 for the oxygen atom, causing strong intermolecular attractions. Surface tension, capillary action, an abnormally high boiling point and even snow flake shapes are due to hydrogen bonds. Lewis Dot Structure of H2O, (Water) Watch on. Chemical Demonstration Videos.

H2O Lewis Structure, Molecular Geometry, and Hybridization
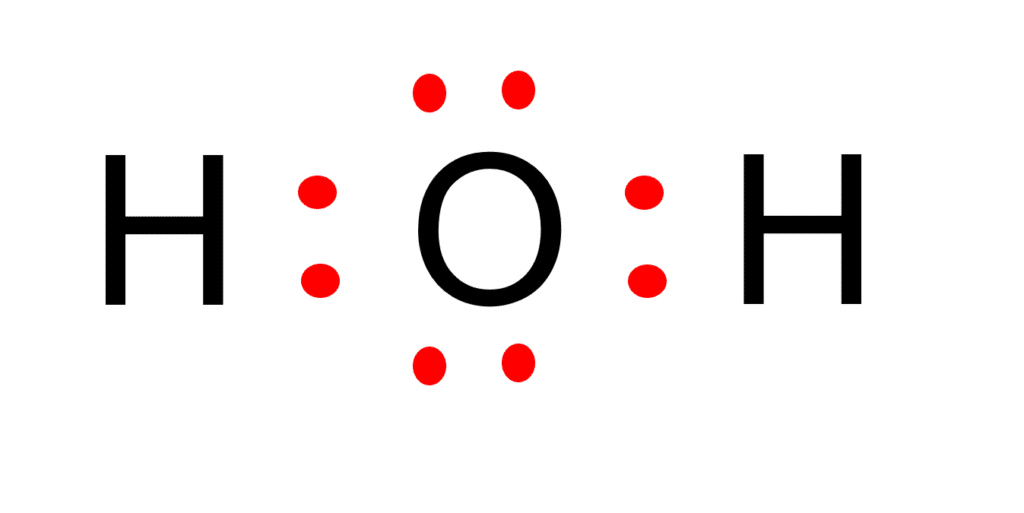
Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons. In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions.
Here, I have explained 6 simple steps to draw the lewis dot structure of H2O (along with images). So, if you are ready to go with these 6 simple steps, then let's dive right into it! Lewis structure of H2O (or Water) contains single bonds between the Oxygen (O) atom and each Hydrogen (H) atom. The Oxygen atom (O) is at the center and it is. The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms, and its electrons. In an H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.
Lewis Structures Hydrogen (H2), and Water (H2O) What's Insight
H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure.This "bent" molecular structure gives it many unique properties such as being polar.One of the most fascinating phenomena is the idea of "hydrogen bonding. Water, with the chemical formula H2O, consists of two hydrogen (H) atoms and one oxygen (O) atom. To create the Lewis dot structure for water, we follow these steps: Determine the Total Number of Valence Electrons: Oxygen has 6 valence electrons (located in the 2s and 2p orbitals), while hydrogen has 1 valence electron each. Therefore, for H2O.




