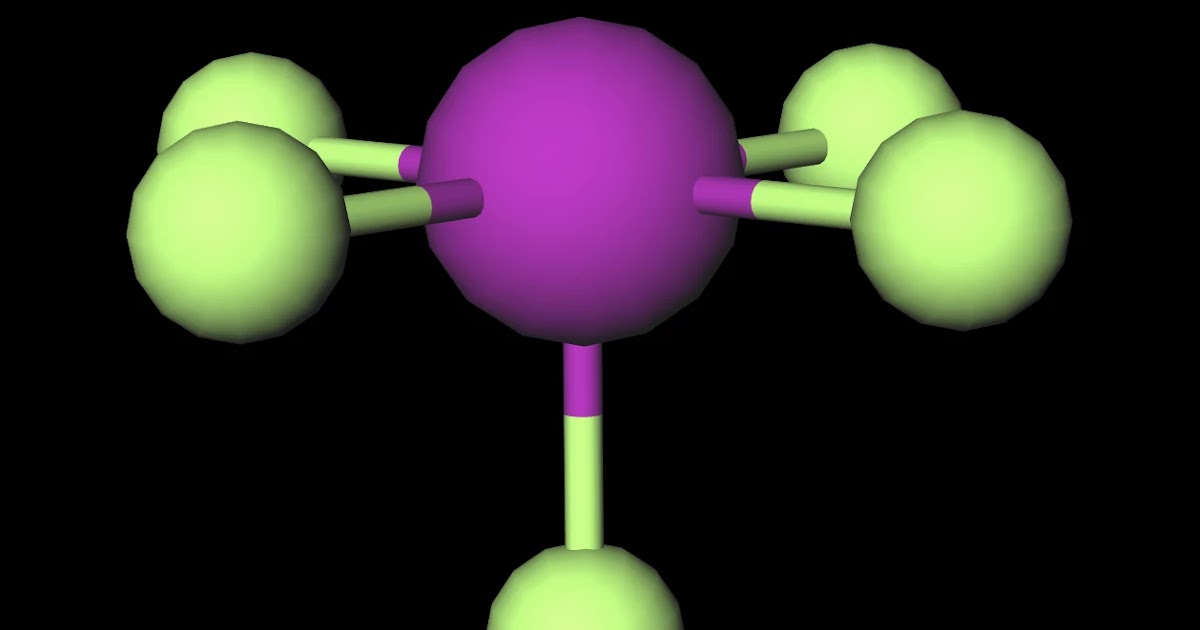
1. The first step is to count all the valence electrons of each molecule. In the case of IF5, The Iodine atom has 7 valence electrons. F also has 7 valence electrons. But since there are 5 atoms of F, we multiply 7×5= 35 valence electrons. Adding both we get 35+7= 42. Hence, a total number of valence electrons of IF5= 42. So, Is IF5 Polar or Nonpolar? IF5 is polar in nature. The molecule has a bent shaped geometrical structure because of lone pair and bond pair repulsion as per VSEPR theory due to which there occurs an imbalance in charge distribution across the molecule.

Is IF5 Polar or Nonpolar? Techiescientist
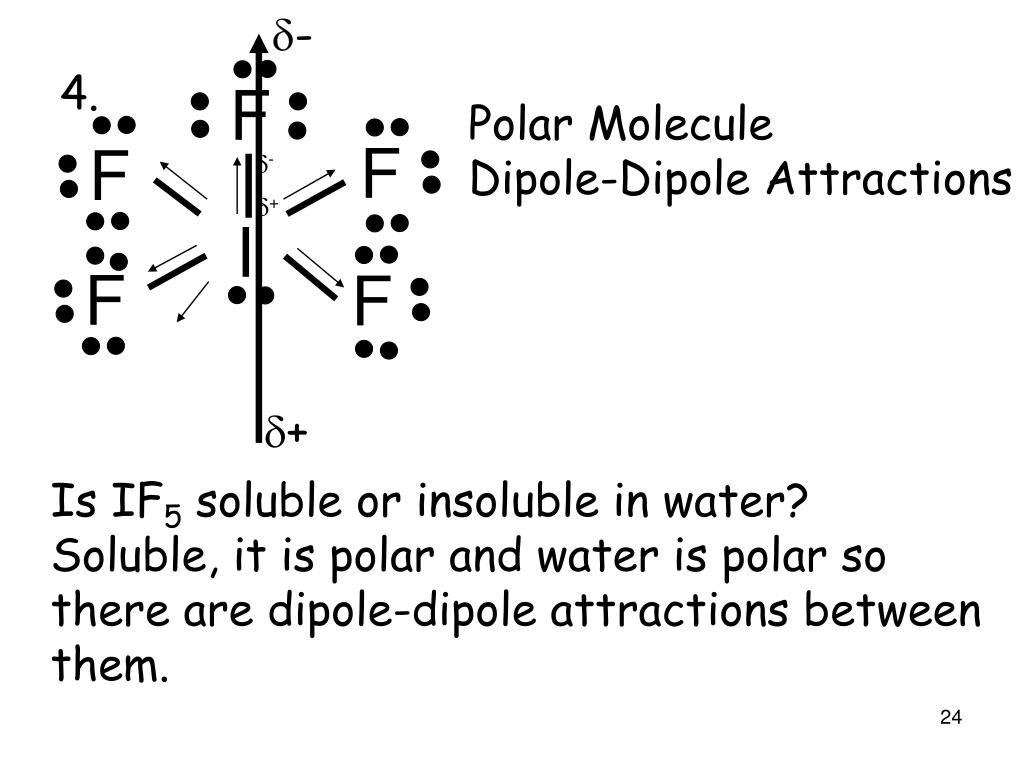
(Explained in 3 Steps) IF5 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of IF5 lewis structure and it contains five I-F bonds. Page Contents show How to draw lewis structure of IF5? The Lewis structure of iodine pentafluoride (IF5) consists of iodine (I) atom at the center. It is bonded to five atoms of fluorine (F) at the sides. There are a total of 6 electron pairs around the central iodine atom in the IF5 lewis dot structure. The Lewis Structure (Lewis Dot Diagram) for IF5.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out. a. SiCl4 b.. | Channels for Pearson+ Next General Chemistry 13. Liquids, Solids & Intermolecular Forces Molecular Polarity 7:17 minutes Problem 52 Textbook Question Determine whether each molecule is polar or nonpolar. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5 Verified Solution 7m
MakeTheBrainHappy Is IF5 Polar or Nonpolar?
Chemistry Chemistry questions and answers Specify whether the molecule IFs is polar or nonpolar and explain why. The molecule is polar because all the I-F bonds are polar and the net dipole moment is nonzero. The molecule is polar only because all the I-F bonds are polar. The molecule is nonpolar because all the I-F bonds are nonpolar. Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule. 100% (11 ratings) Part k The molecule. View the full answer Transcribed image text: Part A Draw an appropriate Lewis structure for IFS. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone Q (5 ଡ BO Identify the geometry of IF5 using VSEPR theory. The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
PPT Part 09 IMFA and Solubility PowerPoint Presentation, free
Organic Chemistry 3h 11m. 23. Chemistry of the Nonmetals 1h 51m. 24. Transition Metals and Coordination Compounds 2h 7m. Predict whether each of the following molecules is polar or nonpolar: (a) IF, (b) CS2, (c) SO3, (d) PCl3, (e) SF6, (f) IF5. Molecules can be classified as polar or nonpolar. The molecule polar behaves in a different manner as compared to nonpolar. Overview: IF5 Lewis Structure. The central atom is Iodine, which is bordered on five terminals with fluorine atoms( in square pyramidal), and one lone pair on the central in the square pyramidal geometry.
Iodine pentafluoride (IF5) is a polar molecule. The central iodine (I) atom in IF5 is surrounded by five fluorine (F) atoms forming a square pyramidal shape. The electronegativity of the fluorine (F) atom is greater than the iodine (I) atom. IF5 has a molecular mass of 221.9 grams per mole and a density of 3.25 g/cm3. It is a melt temperature of -28.8degC, and the boiling point is 47.3degC. The IF5 molecule is polar because of its asymmetrical structure. It comprises an iodine atom centrally bonded to five fluorine molecules in a bipyramidal trigonometric arrangement.

Is If5 Polar Or Nonpolar
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based. There are four electron groups around the central atom. As shown in Figure 9.2.2 9.2. 2, repulsions are minimized by placing the groups in the corners of a tetrahedron with bond angles of 109.5°. 3. All electron groups are bonding pairs, so the structure is designated as AX 4.




