A step-by-step explanation of how to draw the C2H5OH Lewis Dot Structure (Ethanol (Ethyl alcohol)).For the C2H5OH structure use the periodic table to find th. Lewis Structure of C2H5OH. Lewis structure is a representation of all the bonds and lone pairs of different atoms that a compound has. This is a 2-D representation and it helps us to understand more about the properties of the compound. Let's move step-by-step and see how the Lewis Structure of C2H5OH can be made.
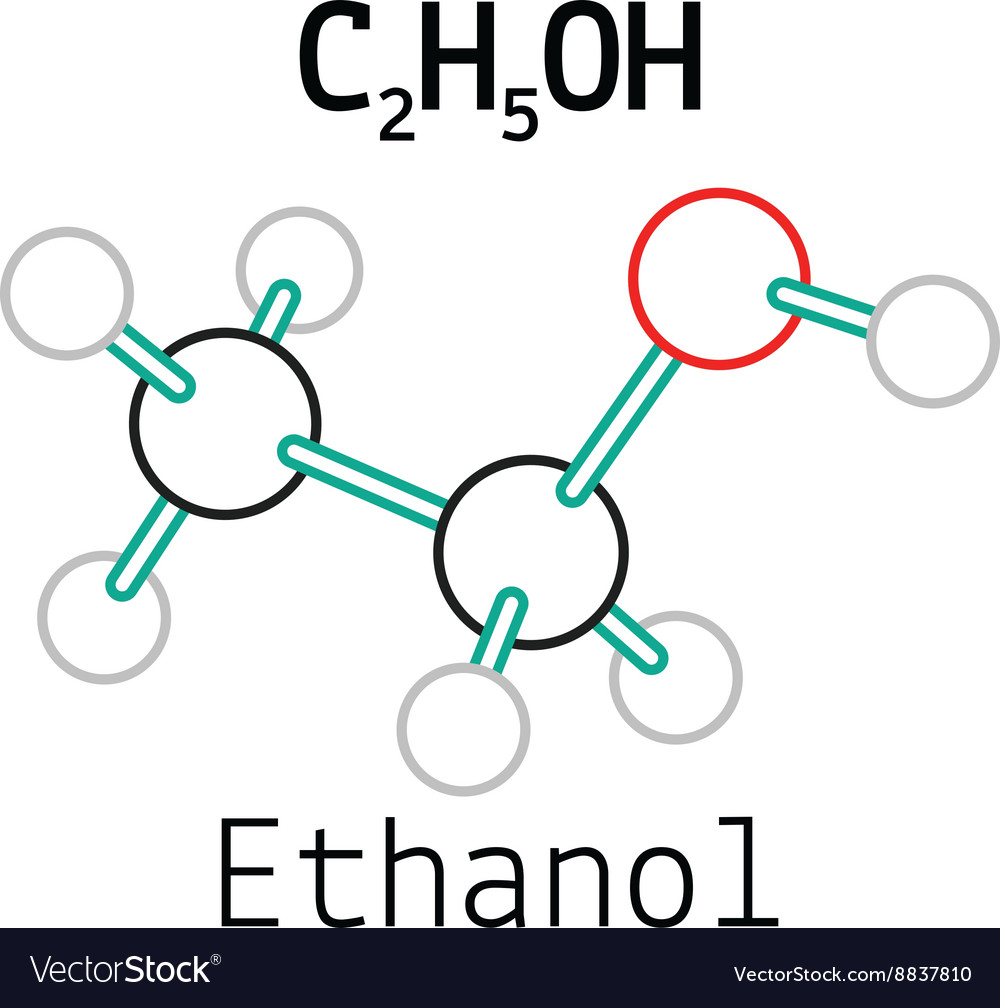
C2h5oh ethanol molecule Royalty Free Vector Image
The Lewis structure is a simple yet powerful tool for understanding the bonding and arrangement of atoms in a molecule. In ethanol, we have a combination of carbon (C), hydrogen (H), and oxygen (O) atoms. To construct the Lewis structure, we start by counting the valence electrons. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1. An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles. Lewis Structure for C2H5OH: https://yout. Hello Guys!In this video, we will determine the Lewis Structure of Ethanol. It has a chemical formula of C2H5OH. To find out its Lewis Structure, we first ca. C2H5OH Lewis Structure, Molecular Geometry, Bond Angles and Hybridization. Written by Priyanka in Lewis Structure. C2H5OH or Ethanol is an organic chemical compound, which can also be represented as CH3-CH2-OH. Ethanol is a colourless liquid with a distinct odour and has a pungent taste.
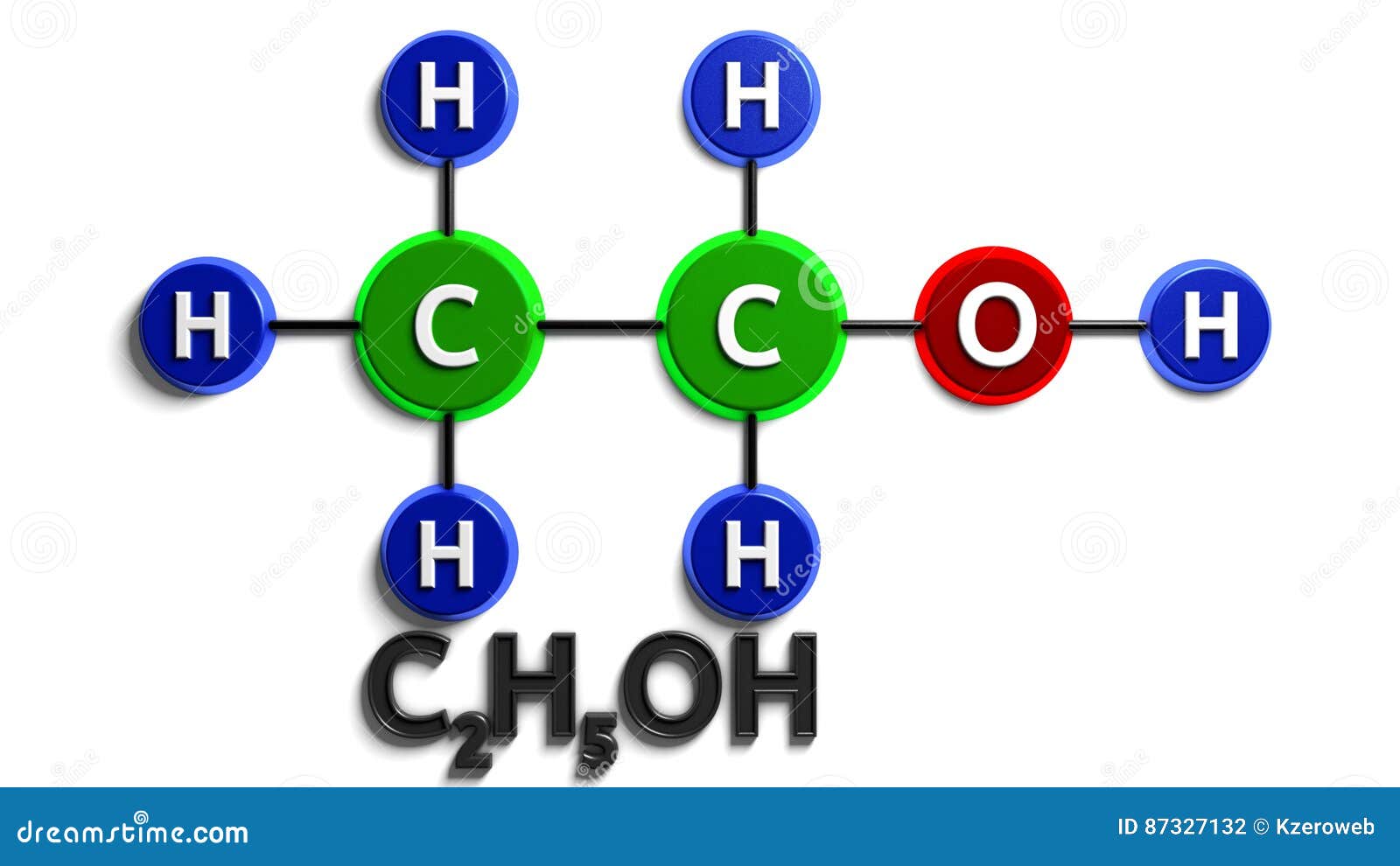
Structural Chemical Formula. Ethanol. C2H5OH. 3d Rendering. Digital
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. Lewis structure of C2H5OH (or Ethanol) contains five C-H bonds, one O-H bond and one C-O bond. The two Carbon atoms (C) are at the center and it is surrounded by Hydrogen atoms (H) and one OH group. The oxygen atom has 2 lone pairs. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history. Steps of drawing C2H5OH lewis structure Step 1: Find the total valence electrons in C2H5OH molecule. In order to find the total valence electrons in a C2H5OH molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Is Ethanol (C2H5OH) Polar or Nonpolar? Techiescientist
Ethanol is a chemical compound that is commonly used as a solvent, fuel, and in the production of alcoholic beverages. Its molecular formula is C2H5OH, and it consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure of ethanol shows the arrangement of these atoms and their valence electrons. The total number of valence electrons available for drawing ethanol (C2H5OH) Lewis structure is 20. C 2 H 5 OH has an identical electron and molecular geometry or shape, i.e., tetrahedral. The C 2 H 5 OH molecule has sp 3 hybridization. The bonded atoms form a mutual bond angle of 109.5° in the tetrahedral C 2 H 5 OH molecule.
Lewis Structure Molecular Formula Condensed Formula Functional Group (s) Compound Type (alkane, alcohol, etc.) 3D Representation (Use lines, wedges, and dashes.) Geometry Around the Carbon Atoms (linear, trigonal planar, or tetrahedral) H CH H | Alkens HC H H2CH2 H Joaca ------ Alkane 354Spahubirea Trigonal planak 46-5p3 Hybridisch Teteanedral. Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
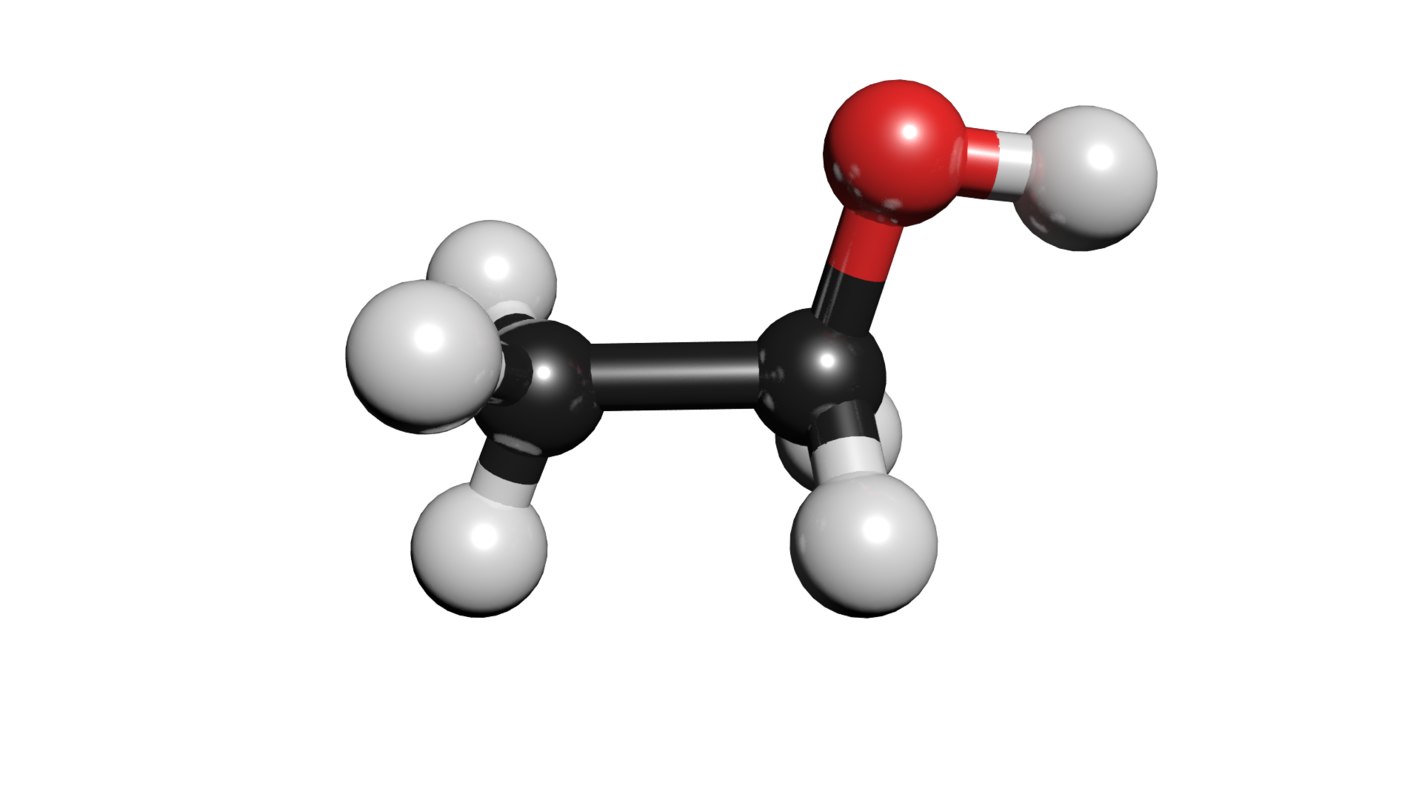
C2h5oh molecule ethanol 3D TurboSquid 1424061
Understand the structure of ethanol and learn to draw it yourself.. C2H5OH, C2H6O, CH3-CH2-OH. Although formulas are written differently, you can see ethanol consists of 2 carbon atoms, 6. Learn to determine if C2H5OH (Ethanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur.




