We can easily draw the Molecular orbital diagram of F 2 following the steps given below. Steps for drawing the molecular orbital (MO) diagram of F2 with its bond order 1. Write down the electronic configuration of F2 atoms F 2 consists of two fluorine (F) atoms. The electronic configuration of each F-atom is 1s2 2s2 2px2 2py2 2pz1. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li 2 to Ne 2. We will predict their bond order and see how the energies of the different orbitals change. We will also compare our predictions to experimental evidence. First, though, we need to talk about a new effect, s-p mixing.
Chemistry Molecular orbital diagrams
Step 1. Start by calculating the number of valence electrons in each atom of F2 and see how many more electrons each fluorine atom needs to form an octet. The atomic number of fluorine is 9; therefore, it possesses 9 electrons in its neutral atomic form. There are 2 electrons in its K shell and 7 electrons in its L shell. Now let's put these ideas together to make an MO diagram for HF. We need to know what orbitals we are using. We are only going to consider valence orbitals. H has a 1s orbital. F has a 2s orbital and 3 2p orbitals (x,y,z). We want to know the energies of the orbitals. Molecular Orbital (MO) Diagram for F2 (2+) 28,328 views 9.5 Molecular Orbital Theory | General Chemistry Chad's Prep When two fluorine atoms bond, the sigma (2p) bonding molecular orbitals are. Molecular Orbital Theory MO bonding in F2 and O2

MO Diagrams for First Row Diatomic Molecules Chemistry LibreTexts
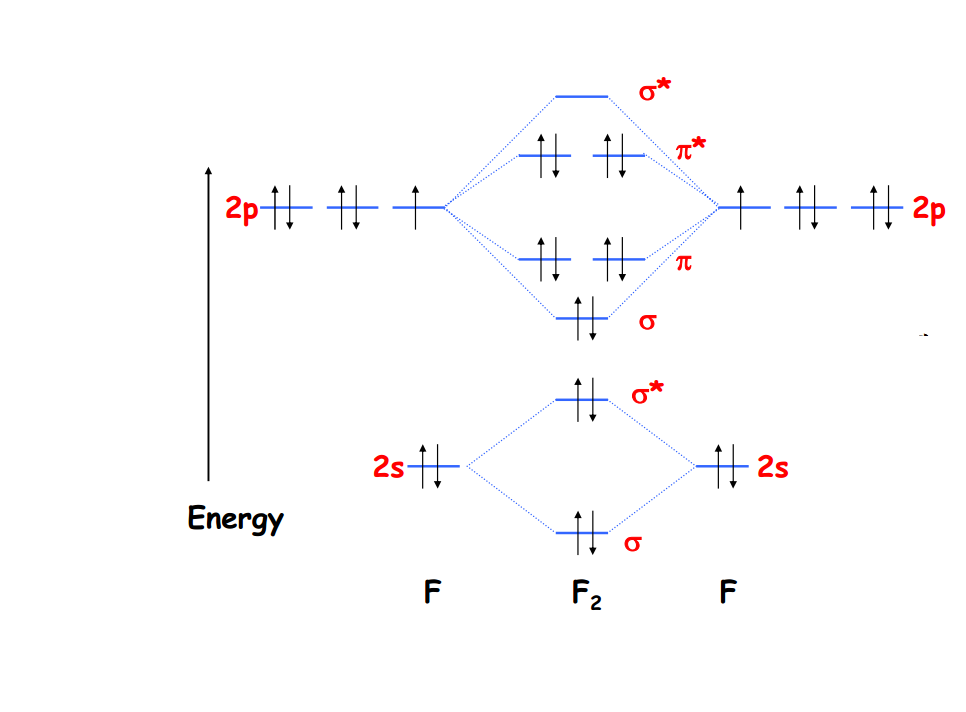
According to our diagram, there are 8 bonding electrons and 6 antibonding electrons, giving a bond order of (8 − 6) ÷ 2 = 1. Thus F 2 is predicted to have a stable F-F single bond, in agreement with experimental data. Figure 9.8.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row: s and p-block Homonuclear Diatomic Molecules. The MO diagram of the valence molecular orbitals can be constructed by combining the valence 2s and valence 2p orbitals from each F atom. The bond order is 1 and the molecule is diamagnetic. This page titled 5.2.1: Molecular Orbitals is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Kathryn Haas . A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.

How to draw Molecular Orbital Diagram for F2 Molecular Orbital Theory
Answer. Exercise 3.3.4.3 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer. 0:00 / 4:36 Molecular Orbital Diagram for F2 and F2+ Brandon C 7 subscribers Subscribed 5.6K views 3 years ago Here is a video that discusses over the Molecular Orbital Diagram for F2+.
For the ion F2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion.————. The interactive videos focused on qualitative quantum physical basics (F1) of the theory as well as practical applications (F2) i.e., the construction and interpretation of so-called MO diagrams to describe bond states between two or more atoms in a chemical compound.

F2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
MO Diagrams for Diatomic Molecules Chapter 5 Friday, October 9, 2015 Homonuclear Diatomic Molecules What happens when we move to more complicated systems? Consider O2. The Lewis dot structure famously predicts the wrong electronic structure for O2 We can use LCAO-MO theory to get a better picture: Draw diagram of forming of sigma and pi bond of C 3 H 4 there is a. The bond lengths are inverse to the bond order, so the order is F2+ < F2 .There is 1 unpaired electron in F2+, 0 unpaired electrons in F2. Draw the \(\sigma\) and \(\sigma^*\) molecular orbital of \(CO\). Draw the MO energy level diagram and write the electron coefficient.



