The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). [7] When the formal charge is non-zero, the S-O bonding is assumed to be delocalized. Figure 9.8.1 9.8. 1: The structure of sulfur dioxide. The modest boiling temperature of SO 2 (-10 °C) means that it is readily liquefied and easily kept as a liquid at room temperature under a slight pressure. The liquid is associated by dipole-dipole attractions due to the polar nature of SO 2. Liquid SO 2 is a good solvent due to the.

SO3 Lewis Structure, Molecular Geometry, and Hybridization
Example 7.3.1 7.3. 1. Transfer the following symbolic equations into word equations or word equations into symbolic equations. HCl(aq) + NaOH(aq) → NaCl(aq) +H2O(l) HCl ( a q) + NaOH ( a q) → NaCl ( a q) + H 2 O ( l) Gaseous propane, C3H8 C 3 H 8, burns in oxygen gas to produce gaseous carbon dioxide and liquid water. Formula: O 3 S Molecular weight: 80.063 IUPAC Standard InChI:InChI=1S/O3S/c1-4 (2)3 IUPAC Standard InChIKey:AKEJUJNQAAGONA-UHFFFAOYSA-N CAS Registry Number: 7446-11-9 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java Javascript . Other names: Sulfur trioxide Henry Agnew (UC Davis) 3.9: Chemical Equations is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. A chemical reaction is the process in which one or more substances are changed into one or more new substances. Chemical reactions are represented by chemical equations. Chemical equations have.. Other names: Sulfur trioxide Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page:. By formula: (CAS Reg. No. 14996-02-2 • 4294967295 O 3 S) + O 3 S = CAS Reg. No. 14996-02-2. Quantity Value Units Method Reference

What Type Of Reaction Happens When Sulfur Dioxide Gas Reacts With
Formula: O 3 S Molecular weight: 80.063 IUPAC Standard InChI: InChI=1S/O3S/c1-4 (2)3 IUPAC Standard InChIKey: AKEJUJNQAAGONA-UHFFFAOYSA-N CAS Registry Number: 7446-11-9 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript . Formula: O 3 S Molecular weight: 80.063 IUPAC Standard InChI:InChI=1S/O3S/c1-4 (2)3 IUPAC Standard InChIKey:AKEJUJNQAAGONA-UHFFFAOYSA-N CAS Registry Number: 7446-11-9 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript . 129 Share 23K views 5 years ago In this video we'll write the correct formula for (Sulfur Trioxide) SO3. To write the formula for Sulfur Trioxidewe'll use the Periodic Table and follow some. Sulfur trioxide. Molecular Formula O. 3. S. Average mass 80.063 Da. Monoisotopic mass 79.956818 Da. ChemSpider ID 23080.

SO3 Molecular Geometry, Bond Angles(Sulfur Trioxide) Molecular
SO3 + H2O → H2SO4 Sulphur trioxide reacts with a base of sodium hydroxide and forms sodium hydrogen phosphate. The chemical equation is given below. SO3 + NaOH → NaHSO4 Uses of Sulphur Trioxide - SO 3 Used as a bleaching agent to remove residual hydrogen peroxide, or as a digesting agent to separate pulp from lignin. Description Sulfur trioxide, is a colorless to white crystalline solid which will fume in air. Often shipped with inhibitor to prevent polymerization. It reacts violently with water to form sulfuric acid with the release of heat. It is corrosive to metals and tissue. It causes eye and skin burns.
Answer link Sulfur assumes it maximum oxidation in sulfur trioxide, SO_3. Sulfur trioxide is the acid anhydride of sulfuric acid: SO_3 (g) + H_2O rarr H_2SO_4 (aq) When sulfur trioxide is dissolved in sulfuric acid, oleum results: SO_3 + H_2SO_4 rarr H_2S_2O_7 Step 3: Converting sulfur trioxide into sulfuric acid. This cannot be done by simply adding water to the sulfur trioxide; the reaction is so uncontrollable that it creates a fog of sulfuric acid. Instead, the sulfur trioxide is first dissolved in concentrated sulfuric acid: \[ H_2SO_{4 (l)} + SO_{3(g)} \rightarrow H_2S_2O_{7 (l)} \label{4}\]
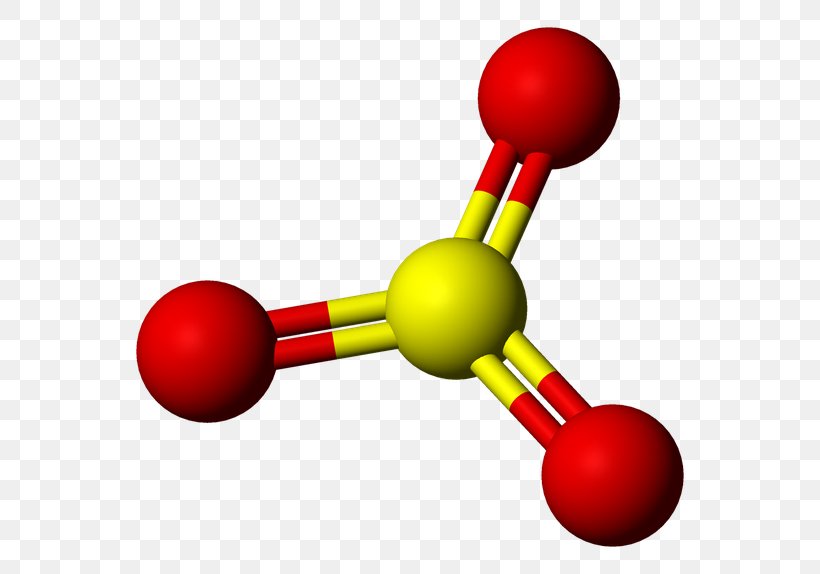
Sulfur Trioxide Molecular Geometry Molecule Sulfur Dioxide, PNG
Instructor Laura Foist View bio Learn what sulfur trioxide is and understand the formula for sulfur trioxide. Find common sulfur trioxide reactions and see sulfur trioxide preparation. Word Equation. Sulfur + Dioxygen = Sulfur Trioxide. S + O2 = SO3 is a Synthesis reaction where two moles of Sulfur [S] and three moles of Dioxygen [O 2] combine to form two moles of Sulfur Trioxide [SO 3] Show Chemical Structure Image. Reaction Type. Synthesis. Redox (Oxidation-Reduction) Reaction.




