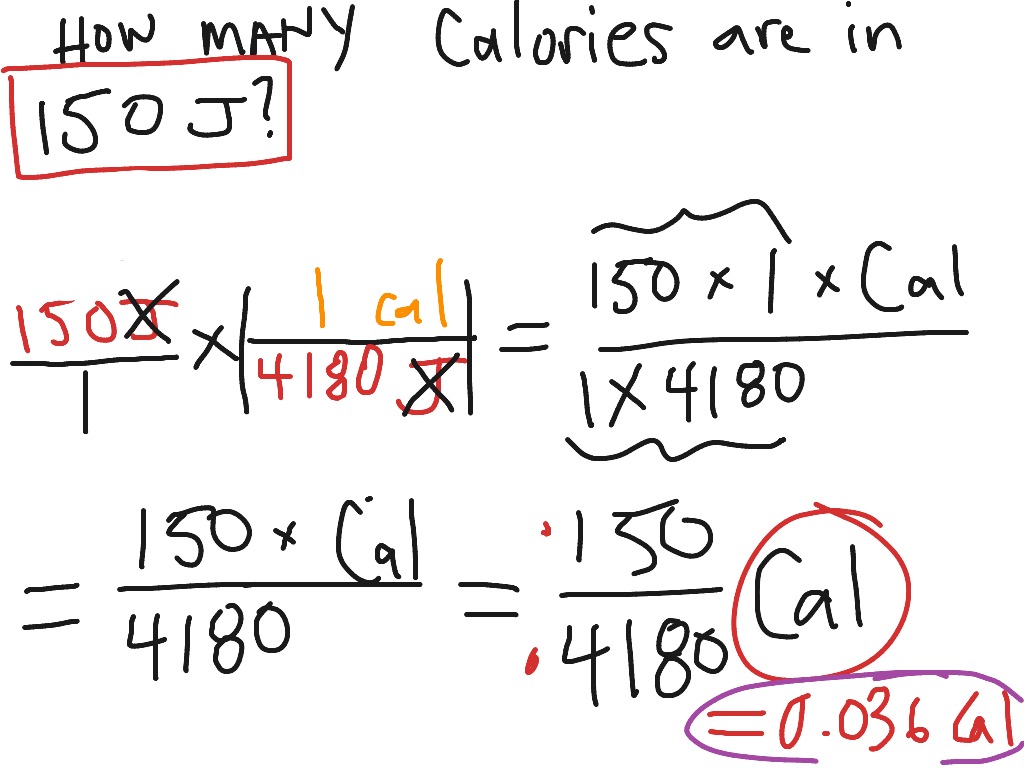How to Convert Calorie (th) to Joule 1 cal (th) = 4.184 J 1 J = 0.2390057361 cal (th) Example: convert 15 cal (th) to J: 15 cal (th) = 15 × 4.184 J = 62.76 J Popular Energy Unit Conversions kJ to kcal kcal to kJ Calories to Joules. Convert between the units (cal → J ) or see the conversion table

16 cal joule YouTube
Joule Definition: A joule (symbol: J) is a derived unit of energy in the International System of Units (SI). It is defined as the energy transferred to an object when a one newton force is applied to the object in the direction of its motion through a distance of one meter. 1 calorie = 4.2 joules 1 Calorie/kcal = 4.2 kilojoules Where, A calorie is the unit of energy. Calorie or kilocalorie is the unit of energy = 1000 calories. Joule is the unit of energy Kilojoule is the unit of energy = 1000 joules. Please note that Calories and calories are not the same. 1 Calorie = 1000 calories. Joules to Calories. Convert between the units (J → cal) or see the conversion table By. Joe Sexton. To convert a measurement in calories to a measurement in joules, multiply the energy by the following conversion ratio: 4.184 joules/calorie. Since one calorie is equal to 4.184 joules, you can use this simple formula to convert: joules = calories × 4.184. The energy in joules is equal to the energy in calories multiplied by 4.184.

how to convert calorie to joule YouTube
There are 0.23900574 calories in a joule. A calorie is measured as the quantity of heat required to raise the temperature of 1 gram of water by 1°C from a standard initial temperature at a pressure of 1 atmosphere. A calorie is also called a "small calorie" or "gram calorie". What is a joule (J)? 1 calorie (cal) is equal to 4.1868 joule (J). 1cal = 4.1868J The Energy E in joule (J) is equal to the Energy E in calorie (cal) times 4.1868, that conversion formula: E(J) = E(cal) × 4.1868 How many Joule in a Calorie? How many cal in 1 joules? The answer is 0.23890295761862. We assume you are converting between calorie [15 °C] and joule . You can view more details on each measurement unit: cal or joules The SI derived unit for energy is the joule. 1 cal is equal to 4.1858 joule. Note that rounding errors may occur, so always check the results. Definitions The "small" calorie is broadly defined as the amount of energy needed to increase the temperature of 1 gram of water by 1 °C (or 1 K, which is the same increment, a gradation of one percent of the interval between the melting point and the boiling point of water).
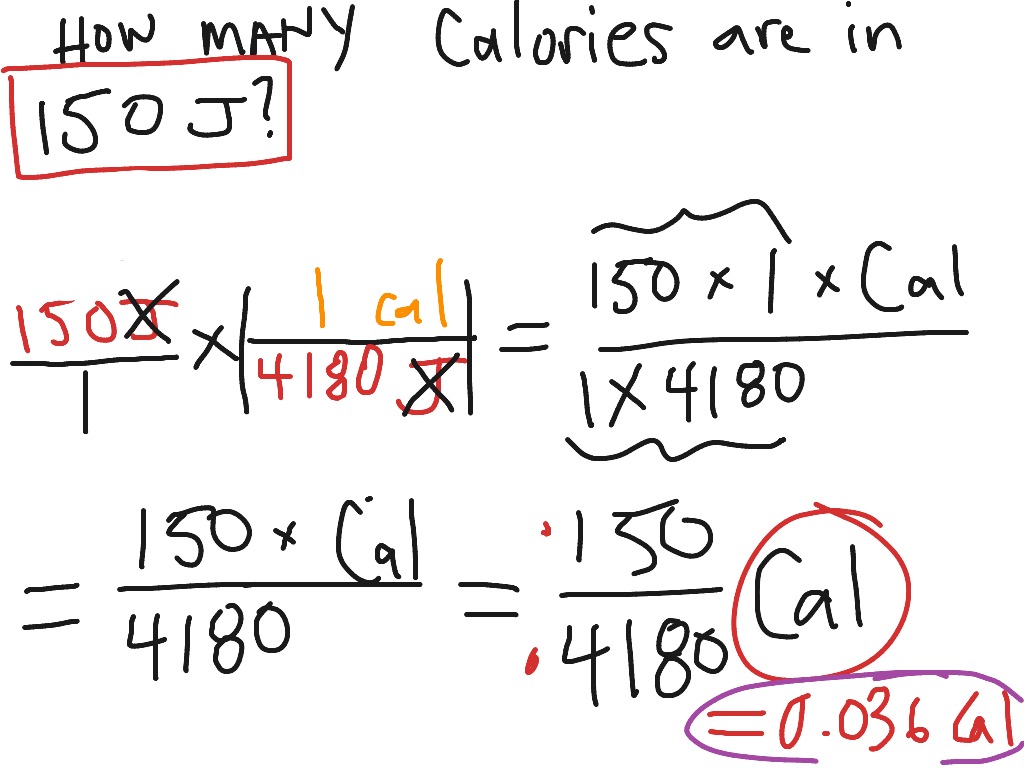
ShowMe Joules
19.4 calorie = 81.1696 joule : 24 calorie = 100.416 joule 34 calorie = 142.256 joule 44 calorie = 184.096 joule 54 calorie = 225.936 joule 64 calorie = 267.776 joule 74 calorie = 309.616 joule 84 calorie = 351.456 joule 94 calorie = 393.296 joule 104 calorie = 435.136 joule 114 calorie = 476.976 joule 124 calorie = 518.816 joule joule More information from the unit converter How many cal in 1 joule? The answer is 0.23890295761862. We assume you are converting between calorie [15 °C] and joule . You can view more details on each measurement unit: cal or joule The SI derived unit for energy is the joule. 1 cal is equal to 4.1858 joule.
Calories. A calorie is the amount of energy it takes to warm 1 gram of water by 1° C. One calorie is equal to 4.184 joules. Joules. One Joule is 1 Newton Metre, ie the work done or energy transfered to an object when a one Newton force acts on it over one metre. Calorie and Joule. 1 Calorie is the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1gram (g) of water through 1 degree Celsius. Joule and Calorie both are units of heat or energy variously defined. The relation between Joule and Calorie can easily be depicted with a simple formula i.e.

Joule Unit
How many Joules are in one Calorie? Thermochemical Calories 1 cal th = 4.184 J 15ºC Calories 1 cal 15 = 4.1855 J Large/food Calories 1 Cal = 4184 J Another unit of energy, used widely in the health professions and everyday life, is the calorie (cal). The calorie was initially defined as the amount of energy needed to warm 1 g of H 2 O by 1°C, but in modern times, the calorie is related directly to the joule, as follows: 1 cal = 4.184 J (7.1.1) (7.1.1) 1 c a l = 4.184 J.