This online quiz is intended to give you extra practice in concepts related to introductory organic nomenclature, including naming compounds and identifying functional groups from diagrams. PLEASE NOTE: structural isomers are not included in this quiz. Select your preferences below and click 'Start' to give it a try! Acceptable names for this molecule include 3-methylhex-2-ene and 3-methyl-2-hexene. IUPAC rules encourage placing the location identifier close to the feature at that location. The first name follows IUPAC rules to the letter. However these names can seem awkward even to chemists, and the second form is used frequently.

Naming Hydrocarbons Practice Worksheet for 10th Higher Ed Lesson
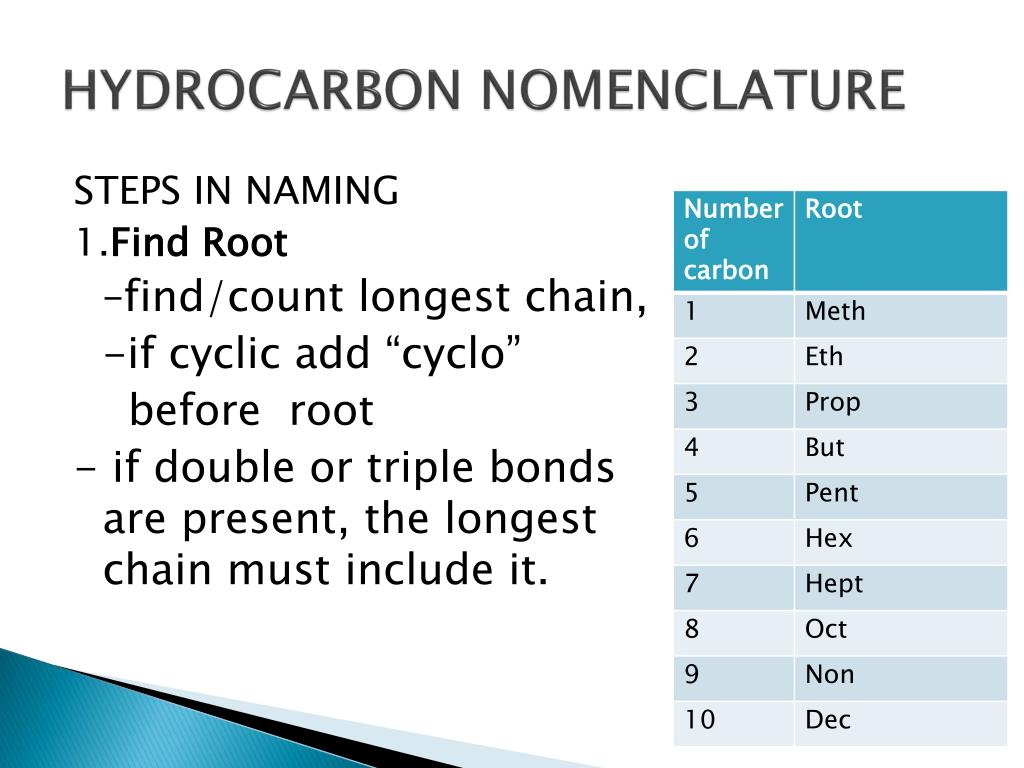
In context worksheets These write-on worksheets will ask learners to use their knowledge of hydrocarbons in an applied context. Calculation questions are included to give opportunities to practise mathematical skills within this topic. There are so many different hydrocarbon molecules possible that a special naming system called IUPAC (International Union of Pure and Applied Chemistry) is used to identify them. This naming system has specific rules so that each molecule has a unique name. How do we Name Hydrocarbons? Nomenclature of organic compounds including hydrocarbons must account for: Functional group (s) and their positions Number of carbon atoms in the longest chain (stem) Type and position of substituents Prefixes for the number of carbon atoms in the longest chain Functional Groups of Hydrocarbons Naming Hydrocarbons Worksheet and Key. Write the name of each of the hydrocarbon molecules shown below: 1) CH2. CH2. CH2. CH3. CH2. CH2.
PPT Naming Hydrocarbons PowerPoint Presentation, free download ID1484308
INTRODUCTION HYDROCARBONS Alkanes Unbranched Chains Unbranched chains Alkenes One double bond More than one double bond E/Z Isomers in Alkenes (iii) Alkynes (iv) Combined Alkenes and Alkynes (v) Cyclic Hydrocarbons COMPOUNDS CONTAINING HALOGENS AND NITRO GROUPS COMPOUNDS WITH FUNCTIONAL GROUPS NAMED AS SUFFIXES General Naming Scheme Answers. A branched hydrocarbon does not have all of its C atoms in a single row. 3-methyl-2-hexene. 4,4-dimethyl-1-pentene. 2,4-dimethyl-2-pentene. 3,4-diethyloctane. 1-bromo-4-chlorobenzene. A unique name can be given to branched hydrocarbons. A unique structure can be drawn for the name of a hydrocarbon. Bookmark Review the rules for naming hydrocarbon structures, including alkanes, alkenes, alkynes and arenes, using this lesson plan with activities for 16-18 year olds In this activity, students assemble the names of hydrocarbon structures using component parts written on cards. This fact sheet introduces IUPAC naming of alkanes, alkenes and alkynes to students, with examples.

Naming Alkanes Worksheet 2
Give the IUPAC name for this compound. Part E: The formula for another cycloalkane derivative is shown below. Give the IUPAC name for this compound. Question 2: Unsaturated Hydrocarbon Nomenclature Part A: The line formula for a branched alkene is shown below. What is the molecular formula of this compound? C H CliffsNotes study guides are written by real teachers and professors, so no matter what you're studying, CliffsNotes can ease your homework headaches and help you score high on exams.
8: Hydrocarbons Structure & Nomenclature: Questions - Chemistry LibreTexts. search Search. build_circle Toolbar. fact_check Homework. cancel Exit Reader Mode. school Campus Bookshelves. menu_book Bookshelves. perm_media Learning Objects. login Login. Choose 1 answer: Hydrocarbons that contain only single covalent bonds between carbon atoms are known as alkynes. A Hydrocarbons that contain only single covalent bonds between carbon atoms are known as alkynes. Hydrocarbons can have the same molecular formula but different molecular geometries. B

Naming Hydrocarbons Worksheets
Practice naming hydrocarbons with branches and rings. Also discusses classification of carbons: primary, secondary, tertiary, and quaternary carbons. Create. Cyclopropane. ethyne. 2,4-dimethylhexane. 2-methylbutane. 3-Ethyl-2-methylhexane. Cyclohexyne. Cyclohexene. Study with Quizlet and memorize flashcards containing terms like butane, but-1-ene, but-2-ene and more.




