Lewis Structure Tutorial A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure (Nitrite ion).For the NO2 - structure use the periodic table to find the total number. This chemistry video tutorial explains how to draw the lewis structure of NO2-, the Nitrite ion.Chemistry - Basic Introduction: https://ww.
NO2 Lewis Structure Nitrite Ion YouTube
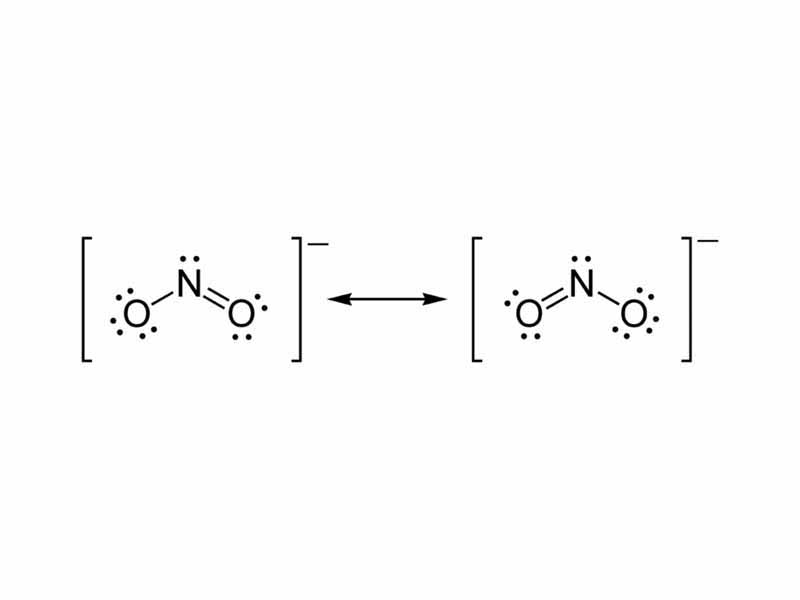
Lewis Structure for NO 2- (Nitrite ion) Lewis structure of NO 2- ion is drawn in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2- lewis structure. Now, we are going to learn, how to draw this lewis structure. Steps of drawing NO 2- lewis structure The nitrite ion, which is NO2 (-1), has two oxygen atoms connected to a central nitrogen atom. To satisfy the octet on nitrogen, exactly ONE of the oxygens needs to be double-bonded to it. But. Lewis Dot of the Nitrite Ion NO 2- Back 70 More Lewis Dot Structures The nitrite ion cannot be satisfactorily represented by just one Lewis Dots structure. All the bonds are the same length and must be thought of as a hybrid of multiple resonance structures. When constructed the NO bond has a bond order of 1.5. In nitrite ion, For Nitrogen we have 5 valence electrons; 6 for Oxygen, but we have two Oxygens so we'll multiply that by two; plus one for this valence electron up here; gives us a total of 18 valence electrons for the nitrite ion Lewis structure Was this answer helpful? 3 Similar Questions Q 1 (i)

Lewis Structure Nitrite Ion Stock Vector (Royalty Free) 2113670753 Shutterstock
Drawing the Lewis Structure for NO 2-(Nitrite Ion) . Viewing Notes: The Lewis structure for NO 2-(Nitrite Ion) comes up quite often in chemistry.; Be sure to put brackets, along with a negative sign, around the NO 2-Lewis structure when you are done to show that it is an ion with a negative charge.; NO 2-has a total of 18 valence electrons. Answer. Example 3.4.2 3.4. 2: Calculating Formal Charge from Lewis Structures. Assign formal charges to each atom in the interhalogen molecule BrCl3 BrCl 3. Solution. Assign one of the electrons in each Br-Cl bond to the Br atom and one to the Cl atom in that bond: Assign the lone pairs to their atom. The Lewi structure of the nitrite ion is presented in figure 2. The structure of nitrite shows that nitrogen is the central atom. It has one pair of lone electrons. Put lone pairs on atoms Stability of lewis structure - Check the stability and minimize charges on atoms by converting lone pairs to bonds. Drawing correct lewis structure is important to draw resonance structures. In another tutorial, we learn how to draw resonance structures of nitrate ion.
Science Image Archive for Teachers
2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. 3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over. General Chemistry: An Atoms First Approach Unit 2: Molecular Structure Chapter 5: Covalent Bonding Chapter 5.3: Lewis Structures Expand/collapse global location Chapter 5.3: Lewis Structures
kentchemistry.com 25K subscribers 226K views 12 years ago Every Video I quickly take you through how to draw the Lewis Structure of NO2- (Nitrite Ion). I also go over hybridization, shape and. Welcome to Warren Institute! In this article, we will dive into the fascinating world of Chemistry and explore the NO2- Lewis Structure. As aspiring

Write the Lewis structure of the nitrite ion, NO2^
Structure Molecular Formula NO2- Synonyms nitrite Nitrite Ion 14797-65- Nitrite anion Nitrous acid, ion (1-) View More. Molecular Weight 46.006 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2005-03-26 Modify: 2023-12-30 Description Nitrites, inorganic, n.o.s. appears as colorless solutions or crystalline solids. 215 110K views 15 years ago A simple way to draw the preferred Lewis electron dot structure for the nitrite anion. I simply draw the valence electrons around each atom and place the extra.




