The ppm to molarity calculator (parts per million) will convert ppm to molarity for any element or molecule dissolved in water. The conversion from ppm to molarity, or back from molarity to ppm, is quite simple. Just read on below to learn more! What is ppm and molarity? Both ppm (parts per million) and molarity are measures of concentration. How to Convert Part/million (ppm) to Milligram/liter 1 part/million (ppm) = 0.9988590004 mg/L 1 mg/L = 1.001142303 part/million (ppm) Example: convert 15 part/million (ppm) to mg/L: 15 part/million (ppm) = 15 × 0.9988590004 mg/L = 14.9828850055 mg/L Convert Part/million (ppm) to Other Concentration - Solution Units
How to calculate ppm per ml mathlasopa
Step 1: Find the Molar Mass of the Substance Look up potassium on a periodic table of the elements. The molar mass is 39.098 g. Because 1 mol of potassium is 39.1 g, by extension, 1 mmol = 39.098 mg. Step 2: Determine the Number of Millimoles Present 500 mL is 0.5 L, and a 0.1 M solution of this volume therefore holds (0.5) (0.1) = 0.05 mol. One part per million (by volume) is equal to a volume of a given gas mixed in a million volumes of air: A micro liter volume of gas in one liter of air would therefore be equal to 1 ppm: Today's more and more there is an interest to express gas concentrations in metric units, i.e. µ g/m 3. Calculate the total parts per million of the solution or substance. Next, determine the molar mass. Measure or calculate the molar mass in g/mol. Finally, calculate the molarity. Calculate the molarity with the equation above. FAQ What is molar mass? Molar mass is a measure of the total mass per mol of substance. For example, PPM means "parts per million". If you find out that the concentration of \text {NO}_2 is equal to 1 ppm, it means that if you take a "sample" of air and divide it into a million parts, one of them will consist of \text {NO}_2. The proportion metrics are as follows: : equal to 1 per 100; : equal to 1 per 1,000,000;
Molarity To Ppm Calculator slideshare
1 Liter of water=1,000 cm^3=1,000,000 mm^3 of water. For example, if we are finding the impurity of Arsenic in 1L of water, we would define it as 1 PPM of Arsenic is present in 1L of water. The parts per million calculator finds the impurity of Arsenic in Per liter of water. The same way we can find the amount of lead (Pb), or Arsenic (Ar. Convert to Molarity FAQs How do you convert molarity to ppm? To convert molarity (M) to ppm (parts per million), you can use the following formula: ppm = (molarity * molar mass) * 10^6 What is 0.1 M in ppm? To convert 0.1 M to ppm, you need to know the molar mass of the solute. 1) Convert to molarity: 2) Convert mol/L to grams of sodium ion per 1000 g of solution: mol/L times 23.00 g/mol x 2 = 0.001104 g/L. 3) Convert to ppm by multiplying numerator and denominator by 1000: 0.001104 g/1000 g of solution times 1000/1000 = 1.104 g / 1,000,000 g of solution. The solution for sulfate is left to the reader. 1 ppm = 1 μg X / ( mL solution)x (1 L/1000 mL) 1 ppm = 1000 μg X / L solution. 1 ppm = 1 mg X/L solution. We know the molarity of the solution, which is in moles/L. We need to find mg/L. To do this, convert moles to mg. moles/L of Cu 2+ = 3 x 10 -4 M. From the periodic table, the atomic mass of Cu = 63.55 g/mol.

Water ppm metric to standard calculator hacbazar
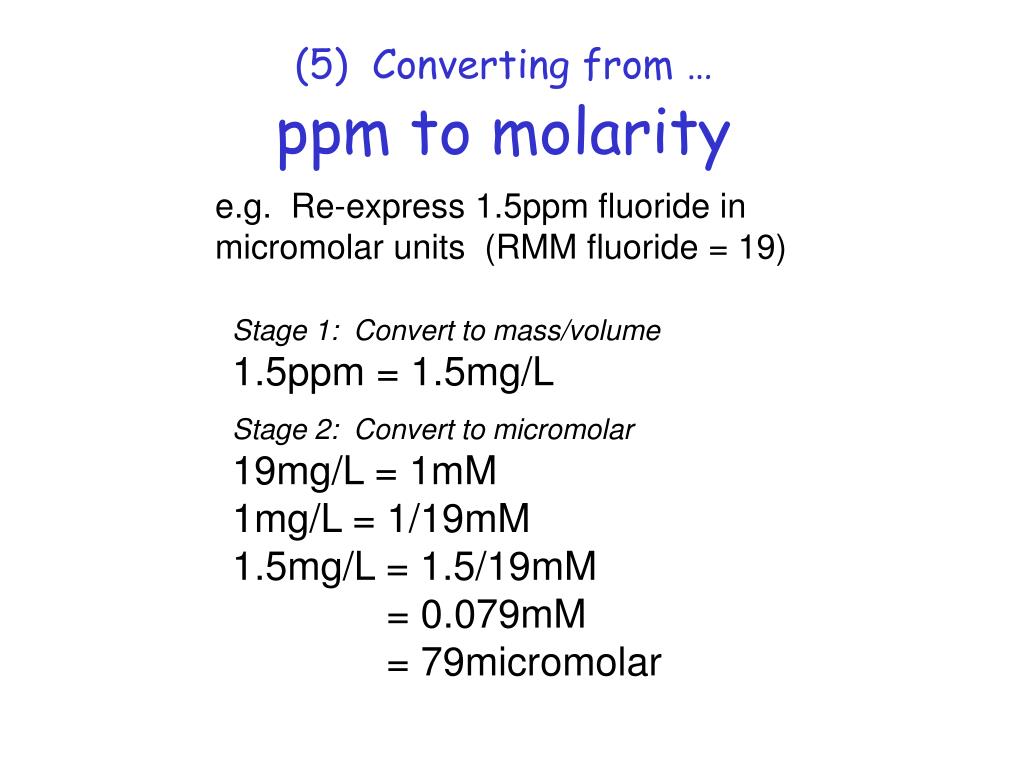
Frequency stability Decimal, percent, permille, ppm, ppb, ppt conversion calculator mg per liter to ppm conversion calculator ppm conversions How to convert ppm to decimal fraction How to convert decimal fraction to ppm How to convert percent to ppm How to convert ppm to percent How to convert ppb to ppm How to convert ppm to ppb Calculate the molarity (mol/L) if there is 64 ppm of Ca2+ ions. And, 40.08g/mol is the atomic weight for calcium ion. Molarity = ( PPM value * 0.001 ) / Atomic Weight. = ( 64 * 0.001 ) / 40.08. = 0.001596806 mol/L. PPM is known as parts per million. Molarity can be expressed as concentration or the number of moles per liter of solution.
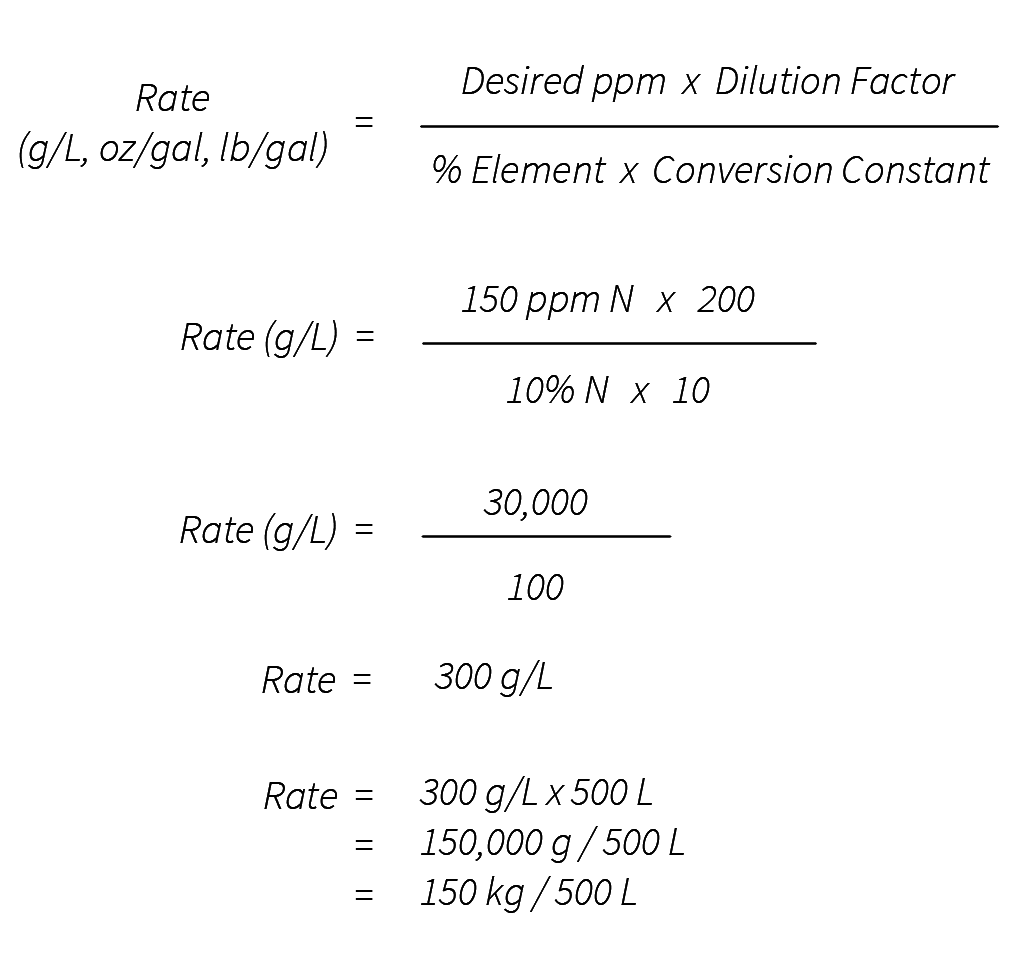
The PPM (Parts Per Million) Calculator is a mathematical tool used to express the concentration of a substance in a solution as a ratio of parts per million. It's widely used in various fields such as chemistry, environmental science, and industry to quantify the amount of a particular component in a larger mixture. The formula for. 1 pound/cubic foot [lb/ft^3] = 0.0160184635 kilogram/liter [kg/L] pound/cubic foot to kilogram/liter, kilogram/liter to pound/cubic foot. Free online concentration - solution converter - converts between 11 units of concentration - solution, including kilogram/liter [kg/L], gram/liter [g/L], milligram/liter [mg/L], part/million (ppm), etc. Also.

How to calculate ppm with molarity beyondlalaf
To convert this value to ppm, multiply it by 1,000,000 (10^6): Salt concentration in ppm = 0.0002 × 10^6 = 200 PPM Similarly, volume calculations can also be used to calculate ppm. Here is a list of elements with their atomic mass which our molarity to parts per million converter uses while doing the calculation. Enter the value of molarity in the below calculator and click calculate button to get the result in ppm. Select Your Element: Actinium [227.028] Aluminum [26.981539] Americium [243] Antimony [121.757] Argon [39..




