1. Physical Chemistry 1.1 Atomic Structure 1.2 Atoms, Molecules & Stoichiometry 1.3 Chemical Bonding 1.4 States of Matter 1.5 Chemical Energetics 1.6 Electrochemistry 1.7 Equilibria 1.8 Reaction Kinetics 2. Inorganic Chemistry 2.1 The Periodic Table: Chemical Periodicity 2.2 Group 2 2.3 Group 17 2.4 Nitrogen & Sulfur 3. Organic Chemistry Complete A level Chemistry Notes Cambridge International AS and A Level Chemistry builds on the skills acquired at Cambridge IGCSE (or equivalent) level. The syllabus includes the main theoretical concepts which are fundamental to the subject, a section on some current applications of chemistry, and a strong emphasis on advanced practical skills.

A Level Chemistry Notes Organic Chemistry Mechanisms (With images
Download File View File AS Chemistry Revision Topical Notes 1 calculations for a-level chemistry by e-n-ramsden.pdf Download File View File cambridge international as and a level chemistry revision guide.pdf Download File View File cambridge-learner-guide-for-as-and-a-level-chemistry.pdf Download File Given the general equation: Hence: Rewriting the equilibrium expression: Assumptions: Ignore the concentration of hydrogen ions produced by the ionisation of the water molecules in the solution, as the ionic product of water (1.00 × 10-14 mol2 dm-6) is negligible compared with the values for most weak acids Alkylamine produced Mechanism: Primary Halogenoalkane (SN2): S stands for substitution, N stands for nucleophilic, 2 is the rate of reaction; depends on both conc. of halogenoalkane and hydroxide ions present. 'Šñm† 'Ä'î 뼕„0Öºiü¿H Û PhŠ$¶nQÅ®"Ð úo @ €-7bF†i;ˆš H…"Üáùés æ¨ »-˜"*Ó¾Wo\ž¸‹h°Ü¹ ÀÈí|°» ·$½,·Sx´( ™Š0ø=û ~Ê( 0éZQ ~|Õ C"ZøÕ£÷ z™ c 0¹+¢ •óK€ Ru é endstream endobj 664 0 obj > endobj 665 0 obj >/MediaBox[0 0 595.32 841.92]/Parent 650 0 R/Resources.

ALevel Chemistry Flash Notes OCR B Year 1 & AS PDF Payhip
1. Moles and equations 2. Atomic structure 3. Electrons in atoms 4. Chemical bonding 5. States of matter 6. Enthalpy changes 7. Redox reactions 8. Redox reactions 9. Rates of reaction A-Level AQA Chemistry Notes Back to Chemistry Revision NEW: Try Revisely's AI Flashcard Generator to automatically transform your notes or textbook into flashcards Filter by Paper General Practical Guide Notes Specification Data Sheet Past Papers Videos Questions by Topic Flashcards Physical 1.1 Atomic Structure Notes Notes Flashcards Welcome to chemistrystudent.com! This is a free website to help students with their learning, studying and revision of A-level Chemistry. Happy Studying! Remember - always think like a proton and be positive. Lets face it, it's only chemistry. A-level Chemistry notes and resources to help students study and revise. CIE Chemistry A Level 4.3.2 Practical Skills for Paper 5 - Analysis, Conclusions and Evaluation Notes www.pmt.education. Analysis, Conclusions and Evaluation Calculations When analysing quantitative data, calculations can be carried out to help summarise and simplify the data.. Notes - Paper 5 Analysis, Conclusions and Evaluation - CIE.

Handwritten A Level Chemistry Notes PDF
he main areas of inorganic chemistry studied include: • redox chemistry • transition elements. 5.1 Rates, equilibrium and Ph Key terms i rate of reaction During a chemical reaction, the concentration of the reactants decreases and the concentration of the products increases. The rate of a reaction is the decrease in Computer Science. 0478. Mathematics. 0580. Chemistry. 0620. French. 0520. Free high-quality revision notes for CIE-AS Chemistry 9701, covering all the modules and updated to the latest syllabus specifications.
Paper 1 Paper 2 Paper 3 Notes: AS Level Practical Paper 3 Notes Theory Section Notes: AS Level: Chapter 1_Atomic Structure Chapter 2_ Atoms, Molecules and Stoichiometry Chapter 3_ Electrons in Atoms Chapter 4_ Chemical Bonding Chapter 5_ States of Matter Chapter 6_ Chemical Energetics Chapter 7_ Redox Reactions and Electrolysis A-Level Chemistry revision notes A-Level (A2/AS) Chemistry revision notes providing information and assistance across all examination boards including AQA, CIE, OCR, Edexcel, Eduqas & WJEC. Chemistry is a complicated A-Level with a lot of content and information to work through.
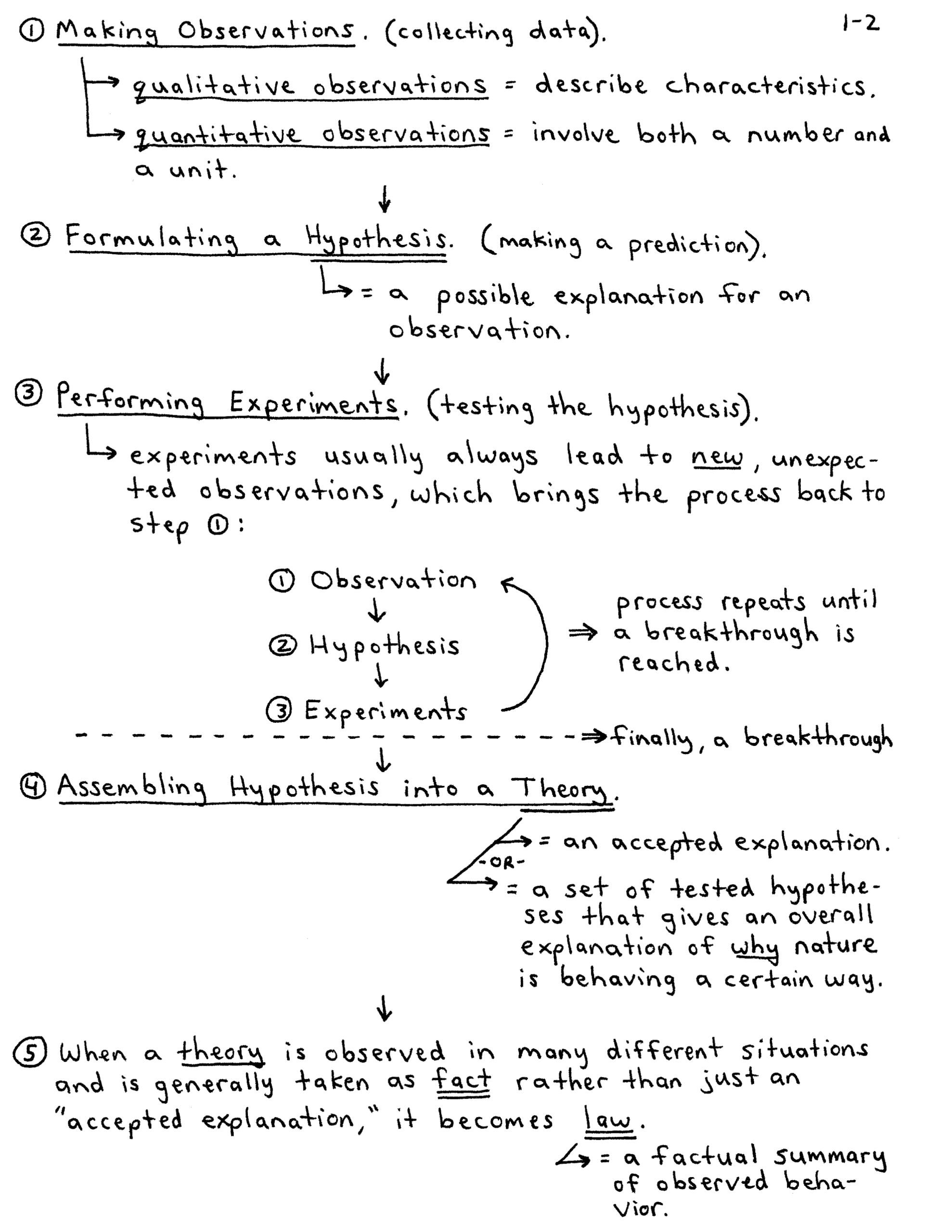
A Level Chemistry Notes PDF Free Download Pdf Keg
An ionic bond is formed when electrons are transferred from a metal to a non-metal, forming an ionic compound. The compound is held together by the electrostatic attraction between the positively charged metal ions and negatively charged non-metal ions. Dot and cross diagrams are often used to represent ionic bonding. Table 1 and table 2 (A level only) show the different types of compounds you are expected to be able to name. A homologous series is a group of compounds with the same functional group, with successive members differing by -CH 2. Table 1: Aliphatic compounds. Homologous Prefix Example series or suffix name. Example molecular formula.




