Rutherford atomic model, nuclear atom, or planetary model of the atom Key People: Ernest Rutherford atom On the Web: UC Davis - The Rutherford Scattering Experiment (Jan. 03, 2024) See all related content → Top Questions What was the impact of Ernest Rutherford's theory? Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. If Thomson was correct, the beam would go straight through the gold foil. Most of the beams went through the foil, but a few were deflected.
Rutherford's atomic model experiment, postulates, limitations & examples
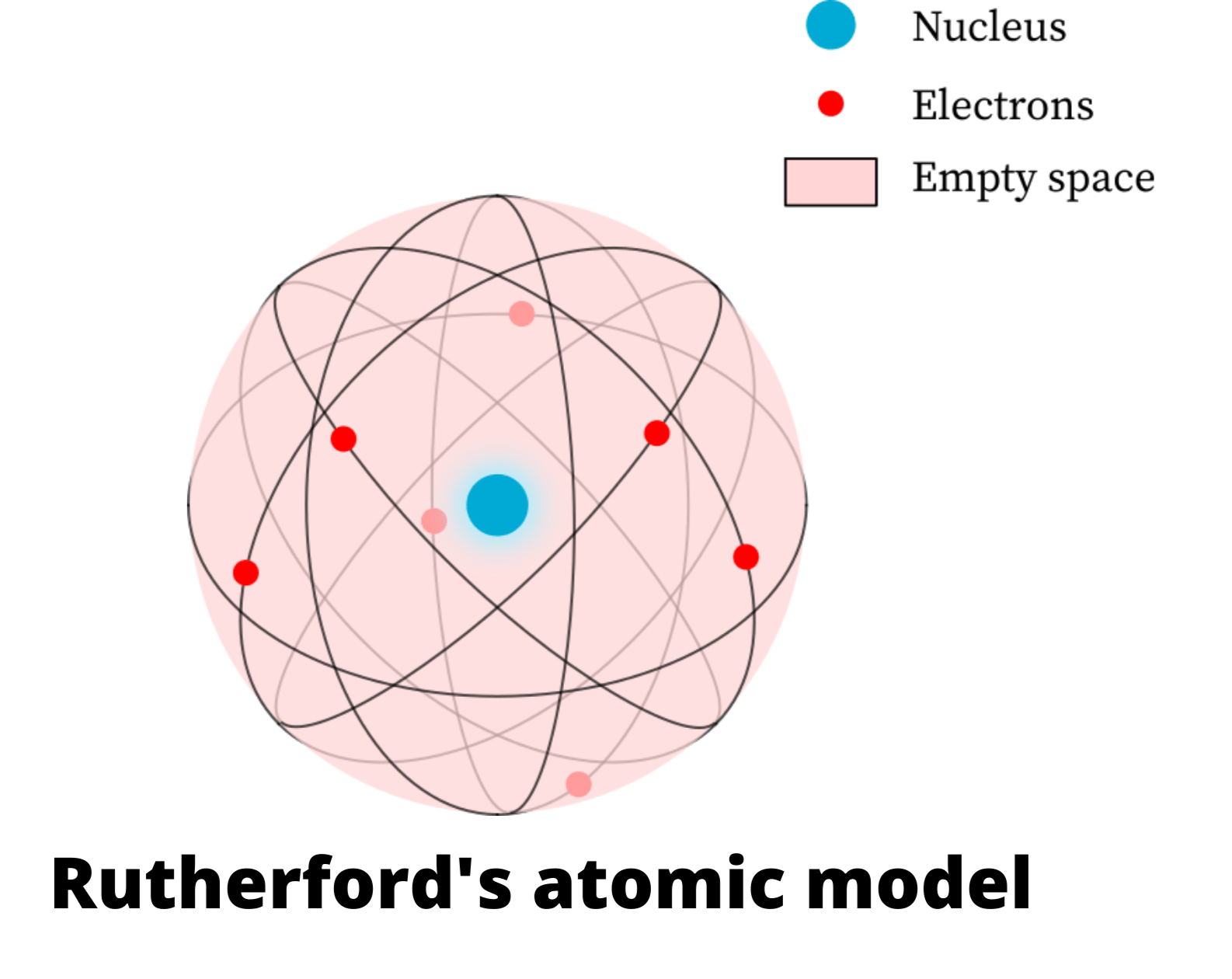
Physicist Ernest Rutherford envisioned the atom as a miniature solar system, with electrons orbiting around a massive nucleus, and as mostly empty space, with the nucleus occupying only a very small part of the atom. The neutron had not yet been discovered when Rutherford proposed his model, which had a nucleus consisting only of protons. (more) Category: Science & Tech In full: Ernest, Baron Rutherford of Nelson Born: August 30, 1871, Spring Grove, New Zealand Died: October 19, 1937, Cambridge, Cambridgeshire, England (aged 66) Awards And Honors: Copley Medal (1922) Nobel Prize (1908) Subjects Of Study: Rutherford model atom radioactivity On the Web: Ernest Rutherford, 1st Baron Rutherford of Nelson, OM, PRS, HonFRSE [7] (30 August 1871 - 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both atomic and nuclear physics. Rutherford has been described as "the father of nuclear physics", [8] and "the greatest experimentalist since Michael Faraday ". [9] Ernest Rutherford (1871-1937) postulated the nuclear structure of the atom, discovered alpha and beta rays, and proposed the laws of radioactive decay. He received the Nobel Prize in Chemistry in 1908. A Series of Discoveries
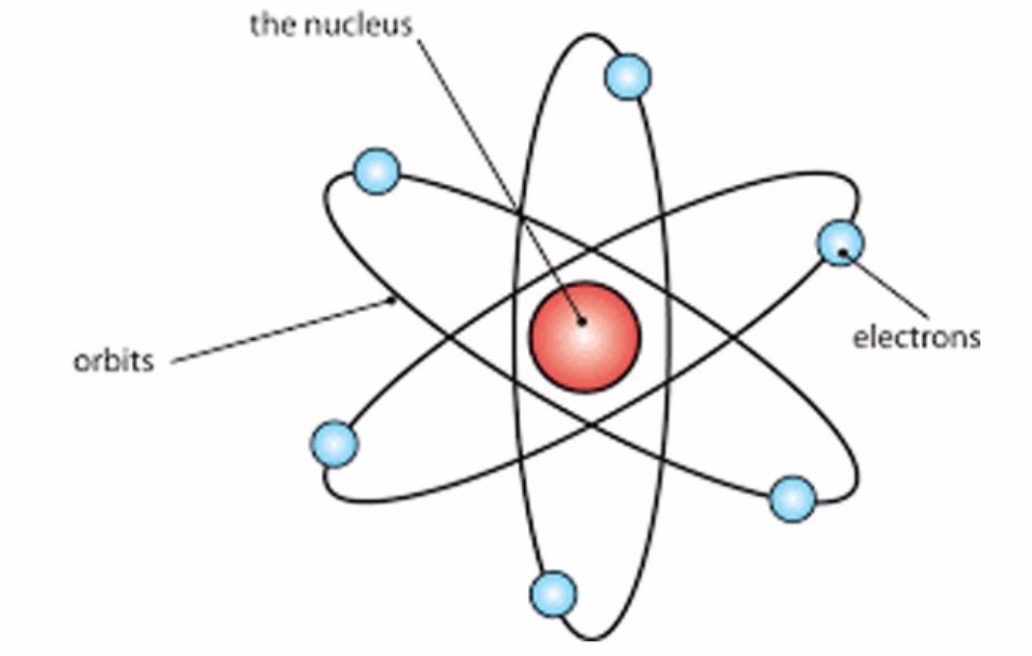
Rutherford Model of an Atom Class 9, Structure of an atom
In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted model of the atom. They bombarded very thin sheets of gold foil with fast moving alpha particles. Figure 3.4.2 3.4. 2 (a) The experimental setup for Rutherford's gold foil experiment: A. Rutherford and the nucleus - Models of the atom - AQA - GCSE Combined Science Revision - AQA Trilogy - BBC Bitesize Home Learn Support Careers My Bitesize More England Early years KS1 KS2 KS3. Rutherford's basic model by proposing that electrons had set energy levels (Fig. 7). This is the model of the atom most commonly portrayed in textbooks: a nucleus orbited by electrons at different levels. It helped solve the problem of the collapsing atom and earned Bohr a Nobel Prize. Just as Bohr built on Rutherford's model, many other In 1913, just two years after the Rutherford atomic model had been introduced, Danish physicist Niels Bohr, a student of Rutherford's, proposed his quantized shell model of the atom (see Bohr model) to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because.
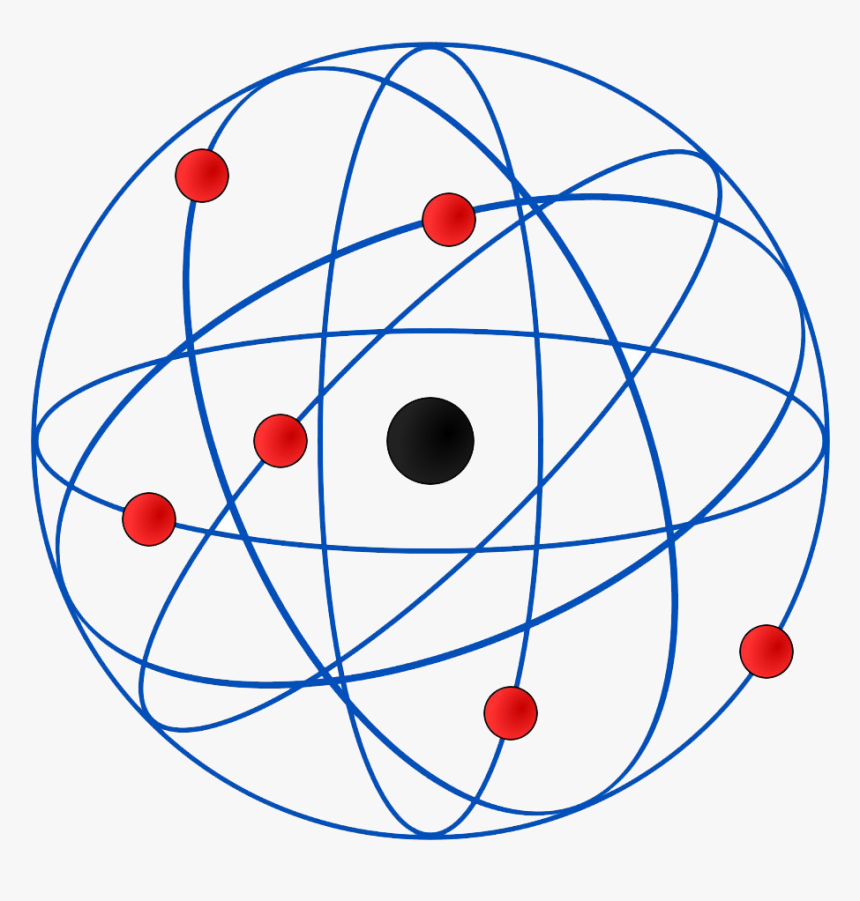
Atom Png Image File Transparent Rutherford Atomic Model, Png Download
Rutherford's Failed Planetary Atom. There are some basic problems with the Rutherford model. The Coulomb force that exists between oppositely charge particles means that a positive nucleus and negative electrons should attract each other, and the atom should collapse. To prevent the collapse, the electron was postulated to be orbiting the. Rutherford's Experiment. In the early 1900's, the plum pudding model was the accepted model of the atom. Proposed in 1904 by J. J. Thomson, the model suggested that the atom was a spherical ball of positive charge, with negatively charged electrons scattered evenly throughout.
This led Rutherford to propose the nuclear model, in which an atom consists of a very small, positively charged nucleus surrounded by the negatively charged electrons. Based on the number of α particles deflected in his experiment, Rutherford calculated that the nucleus took up a tiny fraction of the volume of the atom. Ernest Rutherford discovered the nucleus of the atom in 1911. We read this in textbooks and in popular writings. But what does that statement mean? Geographical discovery usually means that one sees a place for the first time. But can discovery be the same for a realm hidden from sight? One cannot see an atom in that sense.

RUTHERFORD'S MODEL OF ATOM EXPERIMENT,EXPLANATION,PHOTOS,MERITS AND
This page contains materials for the session on the atomic models of Rutherford and Bohr. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.. Bohr's model of the hydrogen atom, Rutherford-Geiger-Marsden. Rutherford's atomic model became known as the nuclear model. In the nuclear atom, the protons and neutrons, which comprise nearly all of the mass of the atom, are located in the nucleus at the center of the atom. The electrons are distributed around the nucleus and occupy most of the volume of the atom. It is worth emphasizing just how small.




