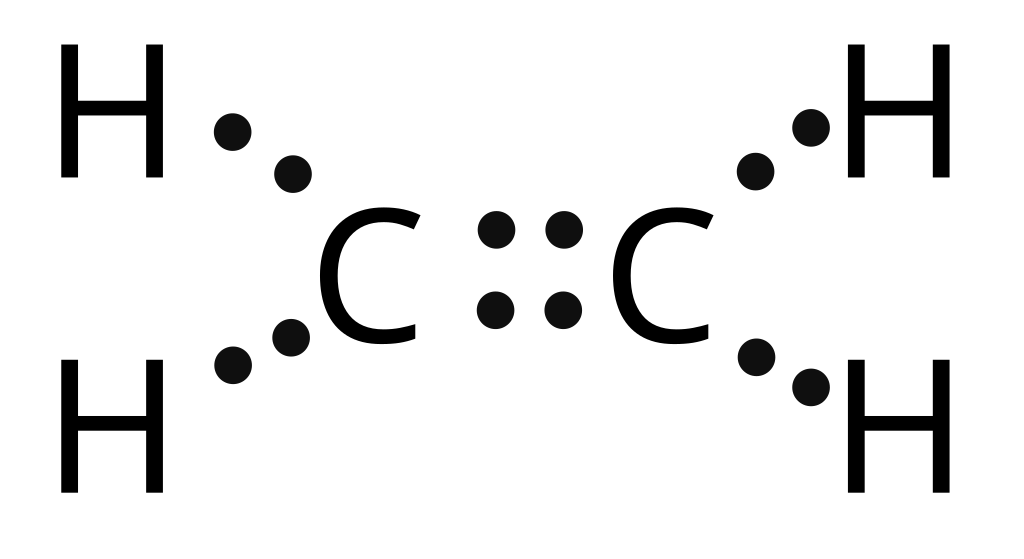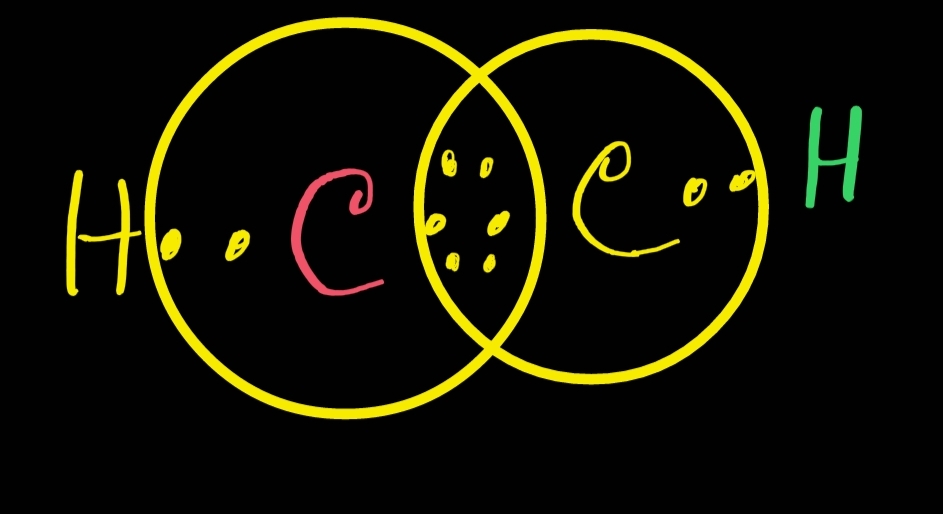2.1K 282K views 10 years ago A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene). For the C2H2 structure use the periodic table to find the total number. Contents show Lewis Structure of Acetylene (C2H2) Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation. To study this, first, it is crucial to know the electronic configuration of the participating elements.
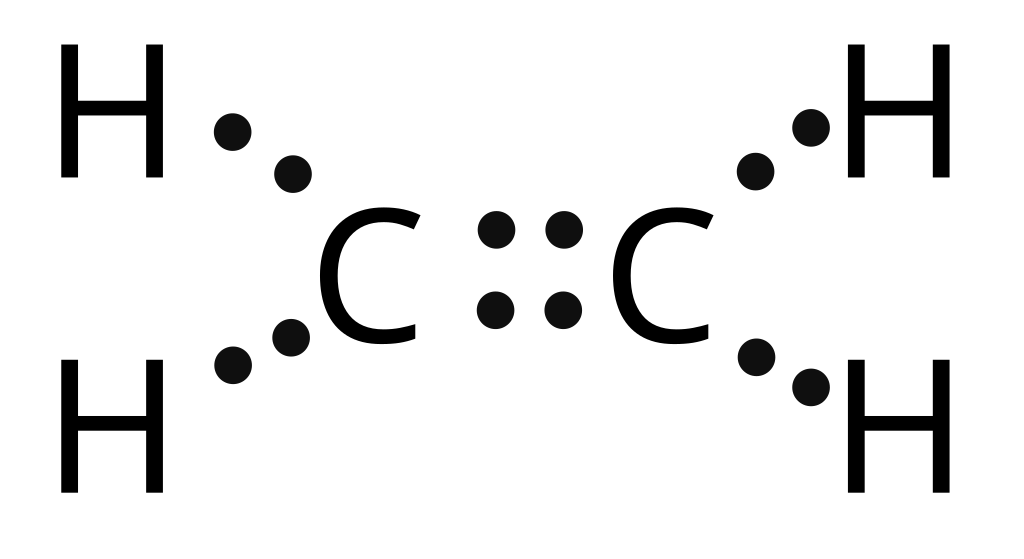
Lewis Dot Diagram For C2h2
27K views 1 year ago A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Acetylene (Ethyne)). For the C2H2 structure use the periodic table to find the total number of. C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step. For C2H2 Lewis structure, we will first place both the Carbon atoms in the centre as it is less electronegative than the Hydrogen atoms. Here both the Carbon atoms take the central position, and the Hydrogen atoms are arranged around it. If you look at the Hydrogen atoms, it only needs one valence electron to attain a stable structure. In drawing the Lewis structure for C 2 H 2 (also called ethyne) you'll find that you don't have enough valence electrons available to satisfy the octet for each element (if you use only single bonds). The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2 .

C2H2 Lewis structure ,Valence Electrons, Formal Charge
Learn the steps to draw the Lewis Structure of C2H2 (ethyne or acetylene) in just 1 minute.📌You can draw any lewis structures by following the simple steps. The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. In the C 2 H 2 Lewis structure, there is a triple bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair. Contents Steps #1 First draw a rough sketch #2 Mark lone pairs on the atoms #3 Calculate and mark formal charges on the atoms, if required By Jay Rana / Last Updated On: June 21, 2023 So you have seen the above image by now, right? Let me explain the above image in short. C2H2 lewis structure has a triple bond between the two Carbon atoms (C) and a single bond between the Carbon atom (C) and Hydrogen atom (H).
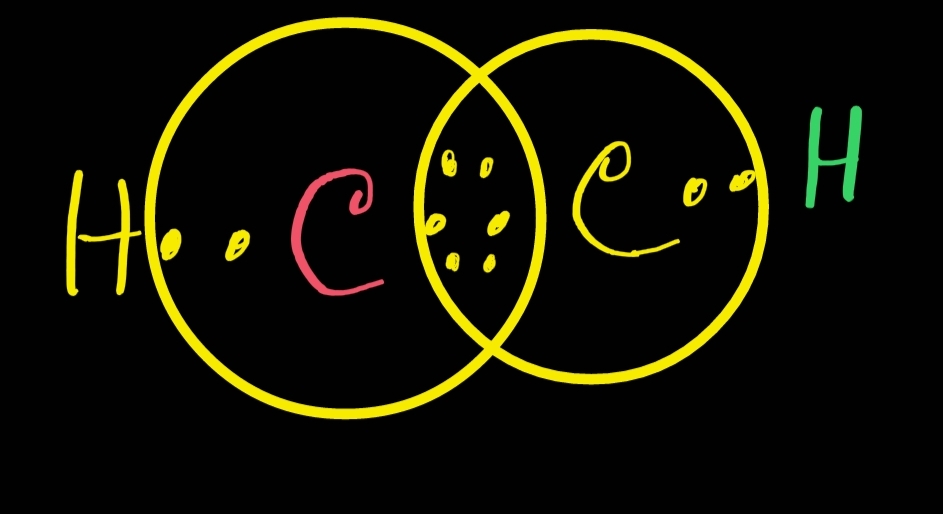
C2H2 Lewis structure ,Valence Electrons, Formal Charge
Lewis structure of C2H2 (or Acetylene or Ethyne) contains one triple bond between the two Carbon (C) atoms and two single bonds between Carbon (C) & Hydrogen (H) atoms. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.
A. Definition and concept The C2H2 Lewis structure refers to the arrangement of atoms and electrons in a molecule of ethyne (C2H2) using Lewis dot diagrams. This involves representing each atom using its chemical symbol and drawing dots around it to represent its valence electrons. C2H2 Molecular Geometry / Shape and Bond Angles (see description for note) Wayne Breslyn 725K subscribers Join Subscribe Subscribed 395 Share Save 121K views 10 years ago A quick explanation of.

C2H2 Lewis structure ,Valence Electrons, Formal Charge
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. C2H2 is a linear molecule in form of geometry and the Lewis structure of C2H2 shows that the carbon atom has four valence electrons, while the hydrogen atom has one valence electron as it is an s-block element. Name of Molecule. Acetylene or ethyne. Chemical Formula. C2H2.