Is SF2 Polar or Nonpolar? Wayne Breslyn 665K subscribers Subscribe 33K views 9 years ago If you look at the Lewis structure for SF2 might appear to be a symmetrical molecule. However, according. SF2 is polar in nature because the sulfur (2.58) and fluorine (3.98) atoms in the molecule differ in their electronegativity and the molecule has a bent geometrical shape. Therefore, the dipoles of the S-F bond do not cancel out each other and molecules turn out to be polar and contribute some dipole moment.
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
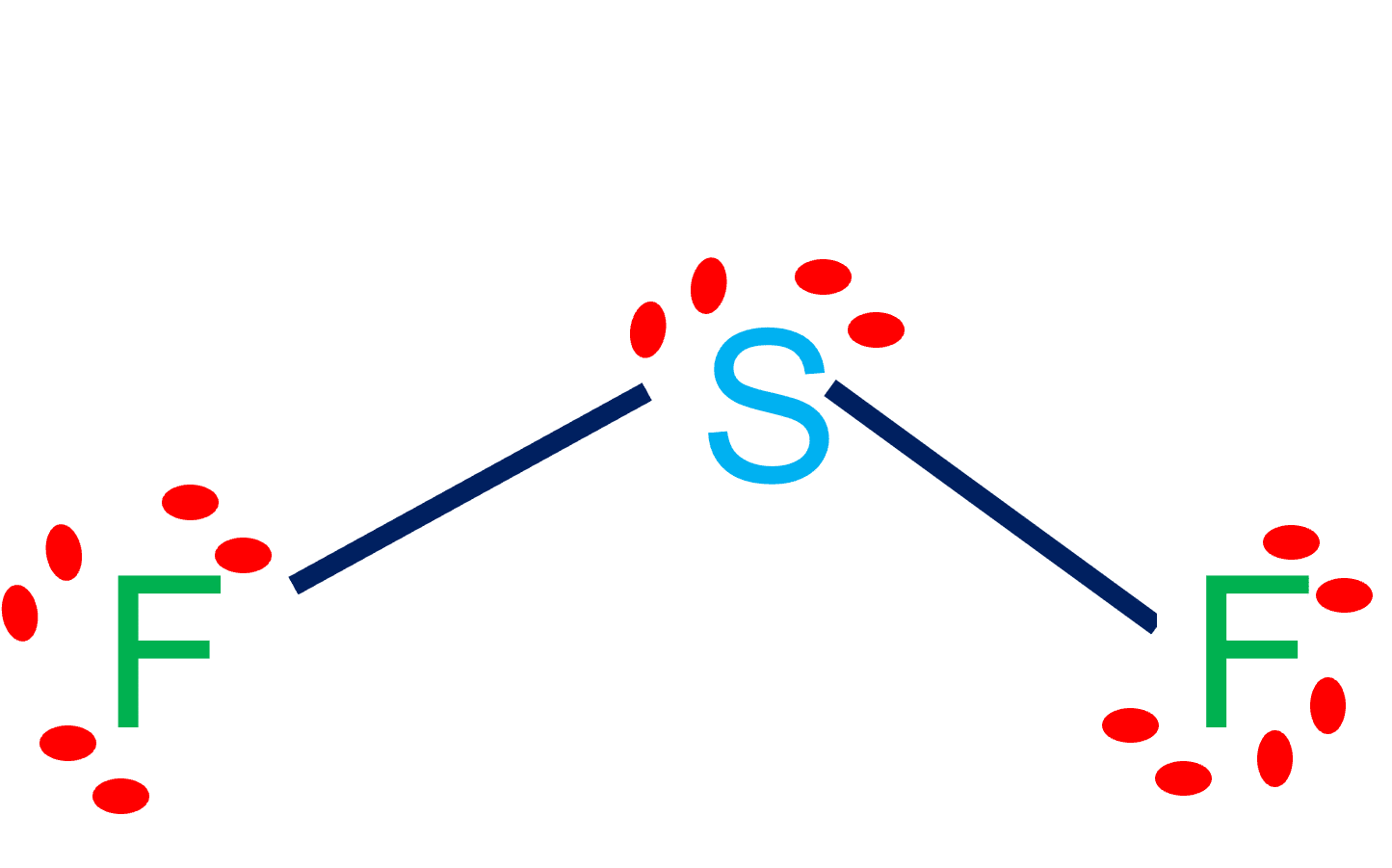
Contents SF2 Valence electrons SF2 Lewis Structure SF2 Hybridization SF2 Molecular Geometry SF2 Shape SF2 Bond Angles Is SF2 polar or nonpolar? SF2 Valence electrons For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. Page Contents show How to draw lewis structure of SF2? SF2 Lewis structure is made up of two atoms, sulfur (S), and fluorine (F), the sulfur (S) is in the central position and fluorine (F) atoms are on either side of it. The lewis structure of SF2 contains 16 nonbonding electrons and 4 bonding electrons. (Explained in 3 Steps) SF2 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of SF2 lewis structure and it contains two S-F bonds. Hey Guys!In this video, we are going to determine the polarity of Sulphur Difluoride having a chemical formula of SF2. It is made of one Sulphur atom and two.

Sf2 polar or nonpolar limfamike
Learn more In this comprehensive guide, we'll walk you through the process of drawing the Lewis structure for SF2, sulfur difluoride. Before we dive into drawing the Lewis structure of SF2, it's crucial to gather some essential information about the molecule. SF2 consists of one sulfur (S) atom and two fluorine (F) atoms. SF2 lewis structure is an electron dot representation which can explain many other characteristics related to it. Discover a step by step method to generate SF2 lewis structure is of significance. How to draw the lewis structure for SF2? Count the number of valence electrons Steps Here's how you can easily draw the SF 2 Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms Now, let's take a closer look at each step mentioned above. #1 Draw a rough skeleton structure First, determine the total number of valence electrons In SF2 lewis structure, the sulfur atom possesses two bonding pairs of electrons and two nonbonding pairs of electrons that reflect the VSEPR idea of AX2E2, which correlates to an angular/non-linear or bent molecular geometry. As a result, Sulfur Difluoride has a bent molecular geometry.

Is SF2 Polar or Nonpolar? Techiescientist
Explain how a molecule that contains polar bonds can be nonpolar. Answer. As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar. PROBLEM \(\PageIndex{2}\) To sketch the SF2 Lewis structure by following these instructions: Step-1: SF2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SF2 for counting valence electrons around the terminal fluorine atoms. Step-3: Lewis dot Structure for SF2 generated from step-1 and step-2.
Step 4: Finally, we need to see if these dipole moments cancel out. In a linear molecule, the dipole moments can cancel out, making the molecule nonpolar. However, in a bent molecule like $\mathrm{SF}_{2}$, the dipole moments do not cancel out. Therefore, the molecule $\mathrm{SF}_{2}$ is \textbf{polar}. Sulfur difluoride (SF2) is a polar molecule. The central sulfur (S) atom in SF2 is surrounded by two fluorine (F) atoms forming a bent or V-shaped molecule. A fluorine (F) atom is more electronegative than a sulfur (S) atom. Thus both S-F bonds are individually polar in the SF2 molecule and possess a specific dipole moment value.

Sf2 polarity furniturevica
Question: Identify each molecule or ion as polar or nonpolar SeF4, BrI5, SF2, CIO3-. Identify each molecule or ion as polar or nonpolar SeF4, BrI5, SF2, CIO3-. There are 2 steps to solve this one. Have you ever wondered whether SF2 is a polar or nonpolar molecule? If so, you're not alone. This question has puzzled many chemistry students and professionals alike. In this article, we'll explore the properties of SF2 and determine once and for all whether it's polar or nonpolar. What is SF2?




