Ethylenediaminetetraacetic acid ( EDTA ), also called edetic acid after its own abbreviation, is an aminopolycarboxylic acid with the formula [CH 2 N (CH 2 CO 2 H) 2] 2. This white, water-insoluble solid is widely used to bind to iron (Fe 2+ /Fe 3+) and calcium ions (Ca 2+ ), forming water-soluble complexes even at neutral pH. 1. Introduction. Divalent metal ions including Mg 2+, Cu 2+, Fe 2+, Mn 2+, Ni 2+, and Zn 2+ play prominent roles in enzymatic catalysis. The small organic compound ethylene diamine tetraacetic acid or commonly known as EDTA is frequently used to chelate these metal ions to investigate enzyme function in the absence of metal co-factors.

EDTA Full Form Ethylene Diamine TetraAcetic Acid StudyWoo
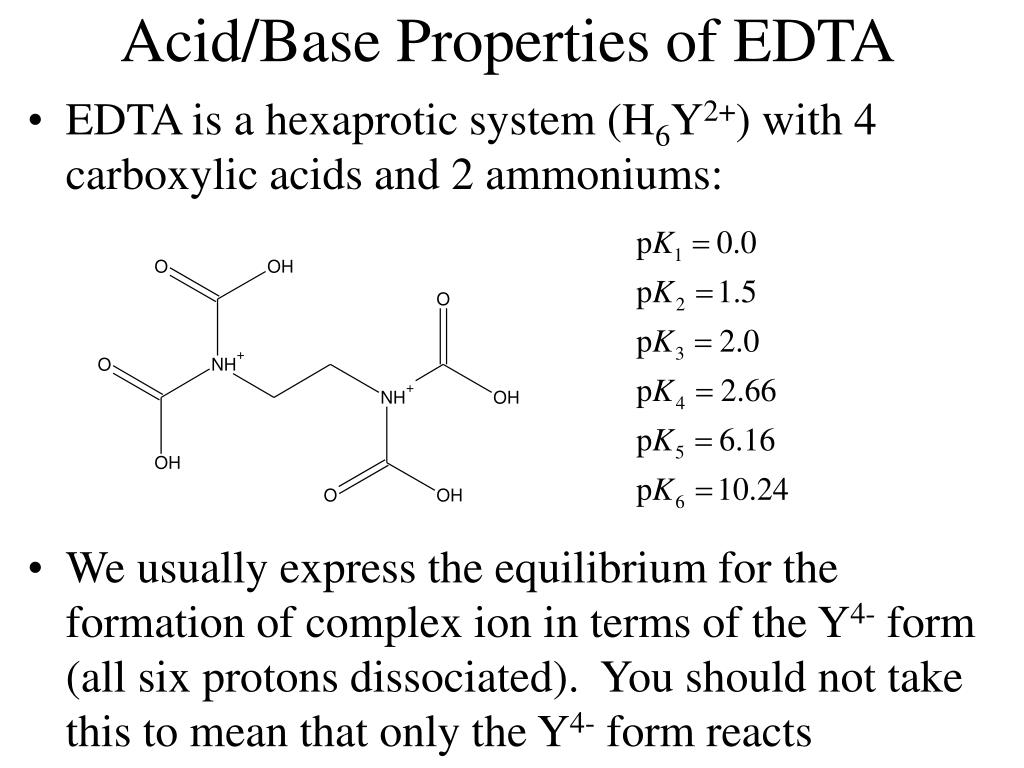
TE buffer is a commonly used buffer solution in molecular biology, especially in procedures involving DNA, cDNA or RNA. "TE" is derived from its components: Tris, a common pH buffer, and EDTA, a molecule that chelates cations like Mg 2+. The purpose of TE buffer is to solubilize DNA or RNA, while protecting it from degradation. Recipe Ethylenediaminetetraacetic acid (EDTA) is a medication used in the management and treatment of heavy metal toxicity. It is in the chelating class of drugs. This activity outlines and reviews the indications, actions, and contraindications for EDTA as a valuable agent in managing lead toxicity. Ethylenediamine tetraacetic acid (EDTA) is a polyprotic acid containing four carboxylic acid groups and two amine groups with lone-pair electrons that chelate calcium and several other metal ions. TBE or Tris/Borate/EDTA, is a buffer solution containing a mixture of Tris base, boric acid and EDTA . In molecular biology, TBE and TAE buffers are often used in procedures involving nucleic acids, the most common being electrophoresis.

EDTA Full Form javatpoint
Chelation Therapy Jeanne A. Drisko MD, in Integrative Medicine (Fourth Edition), 2018 EDTA EDTA is poorly absorbed from the GI tract (<5%) and, as a consequence, should only be administered by a parenteral route. 4 It is primarily distributed in extracellular fluids, which limit its capacity to chelate intracellular metals. EDTA is commonly used as an efficient chelator of metal ion enzyme cofactors. It is highly soluble, optically inactive and does not interfere with most chemicals used in standard buffers making EDTA a common choice to generate metal-free conditions for biochemical and biophysical investigations. However, the controversy in the literature on metal-free enzyme activities achieved using EDTA or. This increased attention to EDTA resulted in studies of EDTA used as an antioxidant occurring in more recent years. The review contained three studies where EDTA was used as an antioxidant in order to treat psoriasis [19-20,22]. Another study in the review utilized EDTA as a chemical treatment in order to cause a decrease of beta lipoproteins . The ADI for humans as set by JEFCA is 1.9 mg EDTA/kg bw (or 2.5 mg Ca-EDTA/kg bw), corresponding to 23.37 mg EDTA/kg bw for mice and is slightly higher than the one we used (21 mg/kg bw) 31.

What is the full form of EDTA? EDTA full form
Popular answers (1) Michael J. Benedik Hamad bin Khalifa University EDTA is a commonly used chelating agent in molecular because it is a very effective chelator of Magnesium. Most nucleases. Biologically important ions such as Ca, K, Mg, Fe, and Zn play major roles in numerous biological processes, and their homeostatic balance is necessary for the maintenance of cellular activities. Sudden and severe loss in homeostasis of just one biologically important ion can cause a cascade of negative effects. The ability to quickly, accurately, and reliably quantify biologically important.
In this chapter, we introduce EDTA (Extensive de novo TE Annotator), a new comprehensive pipeline composed of high-quality tools to identify and annotate all types of TEs. The development of EDTA is based on the benchmarking results of a collection of TE annotation methods. The selected programs are evaluated by their ability to identify true. Applications- EDTA : EDTA is a popular chelating agent for divalent ions, that is widely used in biochemistry, molecular biology and cell biology. EDTA is an abbreviation for EthyleneDiamineTetraAcetic ac (and many other related molecules). EDTA is an amino acid widely used to sequester di- and trivalent metal ions (Ca2+ and Mg2+ for example).
PPT EDTA Titrations PowerPoint Presentation ID234018
Bulk and Prepack available | Ethylenediaminetetraacetic acid disodium salt dihydrate for electrophoresis, for molecular biology |EC Number: 205-358-3; Synonym: Disodium ethylenediaminetetraacetate dihydrate, EDTA-Na2; Linear Formula: C10H14N2Na2O8 · 2H2O | Explore related products, MSDS, application guides, procedures and protocols at Sigma Aldrich - a one stop solution for all your research. It is a popular chemical that goes by several names and is frequently utilized in medicinal and industrial applications. Ferdinand Munz was the first to synthesize this chemical in 1935. It is a colorless, crystalline, slightly soluble organic molecule utilized in biology and inorganic chemistry. It is a chelating agent.




