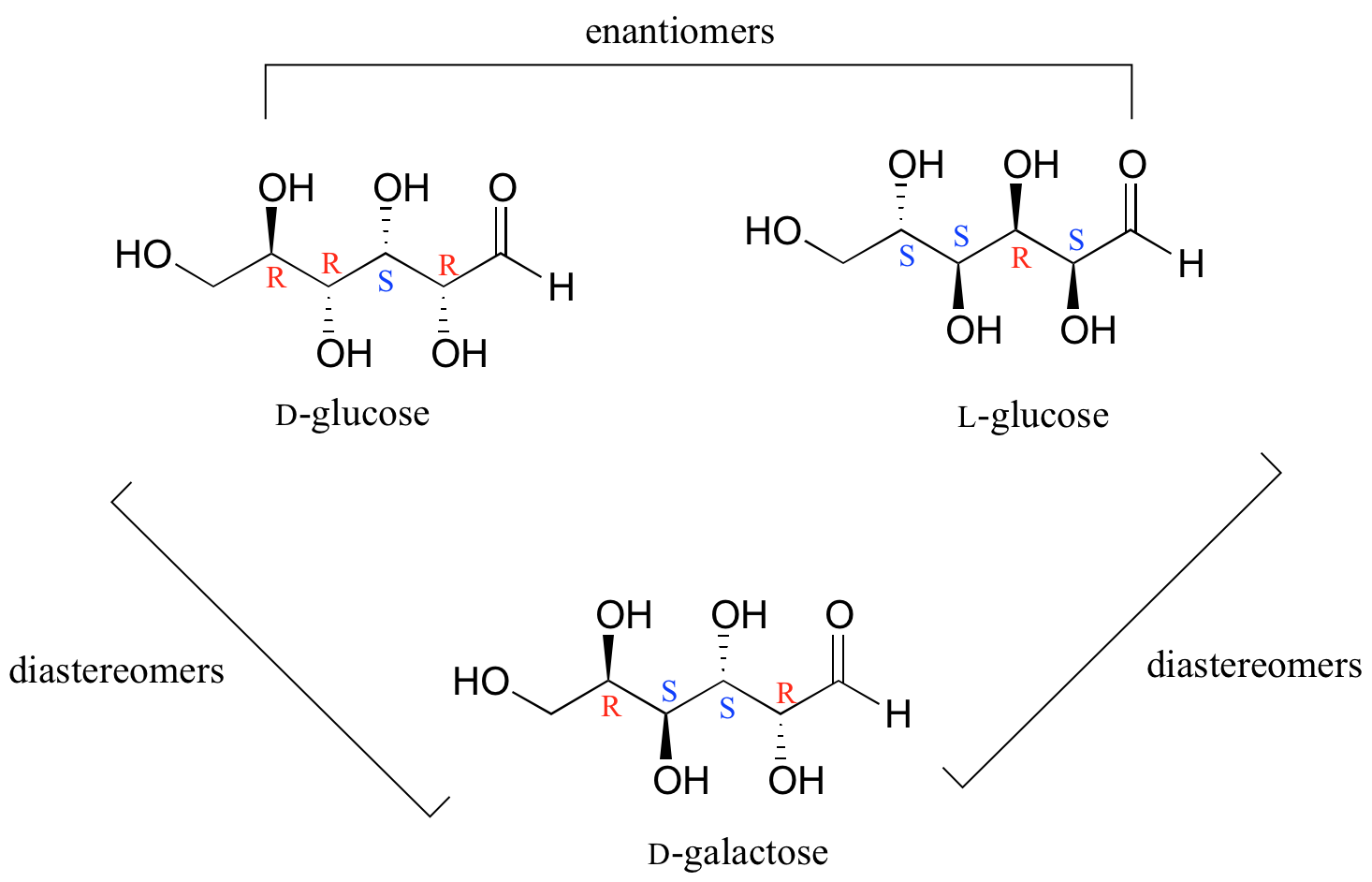
The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves 14 diastereomers of D-glucose: these are molecules in which at least. There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves.

D and LGlucose are enantiomers. Chemistry, Chemistry notes, Science chemistry
There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves. l -Glucose does not occur naturally in living organisms, but can be synthesized in the laboratory. l -Glucose is indistinguishable in taste from d -glucose, [1] but cannot be used by living organisms as a source of energy because it cannot be phosphorylated by hexokinase, the first enzyme in the glycolysis pathway. Thus, L-glucose and D-glucose are enantiomers, but D-Erythrose and D-Threose are diastereomers. Figure \(\PageIndex{1}\): Diastereomers. Figure \(\PageIndex{2}\): Enantiomers. Sugars of 5-7 carbons can fairly easily form ring structures (called Haworth structures). For aldoses like glucose, this involves formation of a hemi-acetal. The D- and L-glucose are true enantiomers. So, enantiomers, which means that they're complete mirror images. They differ at every single chiral carbon. Now that being said, if the D-aldohexoses, these glucose, if the D- and L-aldohexoses are enantiomers, that means that all of the D-aldohexoses have to be diastereomers of each other, because.

CH103 Chapter 6 Natural Products and Organic Chemistry Chemistry
D- and L-is an old but still-convenient shorthand for saying that molecules are enantiomers. e.g. D-glucose and L-glucose are non-superimposable mirror images without having to write out a long IUPAC name with lots of ( R) and ( S) descriptors. Most natural sugars are D- and most natural amino acids are L- . Enantiomers are a pair of molecules that exist in two forms that can not be superimposed on each other but are mirror images of each other. They have a chiral carbon which is a center of carbon. Enzyme-free substrate is used for SERS sensing glucose enantiomers. • Au NPs play as the oxidase mimics instead of the SERS substrate. • Intrinsic structure of MOFs is favorable for applying as nanoreactors. • Enantioselective identification is achieved via onsite growth of Prussian blue. • This platform is also useful for other. 1 Answer Maxwell Aug 12, 2016 They are not enantiomers. They are diastereomers. Explanation: Diastereomers are molecules that have 2 or more stereogenic centers and differ at some of these centers with respect to absolute configurations. This disqualifies them from being mirror images of each other.

Adisi Nukleofilik
There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves. Although sugar enantiomers may display the same or similar biological activity, it is obvious that the protein-binding properties of a d -sugar component of a lead synthetic glycoside are different from its L-enantiomer (or vice versa).
There are three common naming conventions for specifying one of the two enantiomers (the absolute configuration) of a given chiral molecule: the R/S system is based on the geometry of the molecule; the (+)- and (−)- system (also written using the obsolete equivalents d- and l-) is based on its optical rotation properties; and the D/L system is based on the molecule's relationship to. Their enantiomers were given the same name with the introduction of systematic nomenclatures, taking into account absolute stereochemistry (e.g. Fischer nomenclature, d / l nomenclature). For the discovery of the metabolism of glucose Otto Meyerhof received the Nobel Prize in Physiology or Medicine in 1922. [16]
5.8 Diastereomers Chemistry LibreTexts
For example, let's consider the glucose molecule in its open-chain form (recall that many sugar molecules can exist in either an open-chain or a cyclic form). There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore. Q1 What are Epimers with examples? Epimers are carbohydrates that differ in the location of the -OH group in one location. Both D-glucose and D-galactose are the best examples. D-glucose and D-galactose epimers create a single difference at C-4 carbon. They are not enantiomers, they are just epimers, or diastereomers, or isomers. Q2




