5.14: Hydrogen, Helium, Lithium. With some familiarity with the properties of single electrons, such as the single electron around the hydrogen nucleus above, we can discuss atoms containing more than one electron. The images found here depict electron wave density by number of dots. Thus, more dots indicates more electron density 'cloud' in. nuclear astrophysics High-energy nuclear physics Scientists Physics portal Category v t e Nuclear fusion is a reaction in which two or more atomic nuclei, usually deuterium and tritium (hydrogen variants), combine to form one or more different atomic nuclei and subatomic particles ( neutrons or protons ).
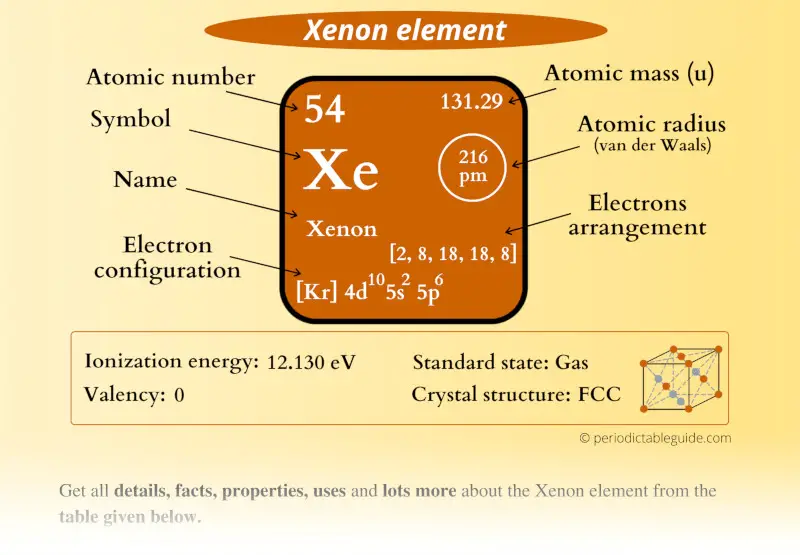
Xenon (Xe) Periodic Table (Element Information & More)
Therefore, only hydrogen, helium and lithium were available when the earliest stars formed several hundred million years later. As we have seen, almost all of this material was 1 H and 4 He. The reactions that could form the lightest elements beyond hydrogen and helium are shown in Figure 16.8. The formation of beryllium is speculative, as we. Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups. Hydrogen is the most abundant element in the Universe; helium is second. However, after this, the rank of abundance does not continue to correspond to the atomic number; oxygen has abundance rank 3, but atomic number 8. All others are substantially less common. Figure 6.19.1 6.19. 1 The figures above show the electron density of different elements. On the left from top to bottom are Hydrogen, Helium, and Lithium. On the right from top to bottom are Beryllium, Boron, and Carbon. Hydrogen has a larger circular area concentrated with dots when compared to helium.

1 H Hydrogen 2 He Helium 3 Li Lithium 4 Be
The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. The atomic number of an element provides insight into the number of protons that exist inside the nuclei of the atoms of that element and also into the number of electrons that surround these nuclei. For example, the atomic number of sodium is 11. Lithium is rare in the Universe, although it was one of the three elements, along with hydrogen and helium, to be created in the Big Bang. The element was discovered on Earth in 1817 by Johan August Arfvedson (1792-1841) in Stockholm when he investigated petalite, one of the first lithium minerals to be discovered. for allowed and forbidden lines of hydrogen, helium and lithium, including Li II, as well as the hydrogen isotopes deuterium and tritium. Altogether, we tabulated about 3600 transitions and listed scaling relations for the hydrogenlike ions He II and Li III. The selected data are based on a critical evaluation of available literature sources.

Hydrogen, helium and lithium atoms, illustration Stock Image C050/7595 Science Photo Library
The periodic table is a chart that organizes the elements by their atomic number. The first 30 elements are hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon, sodium, magnesium, aluminum, silicon, and phosphorus. You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences.
Hydrogen has no neutrons, helium has two, lithium has four and beryllium has five, and the masses of the elements increase in that order. Hydrogen and helium are gases, whereas lithium and beryllium are metals. The Periodic Table and the Masses of Elements You can easily identify the lightest elements by checking the periodic table (see Resources). Example 1: Helium vs. Lithium. Hydrogen has an electronic structure of 1s 1. It is a very small atom, and the single electron is close to the nucleus and therefore strongly attracted. There are no electrons screening it from the nucleus and so the ionization energy is high (1310 kJ mol-1). Helium has a structure 1s 2. The electron is being.
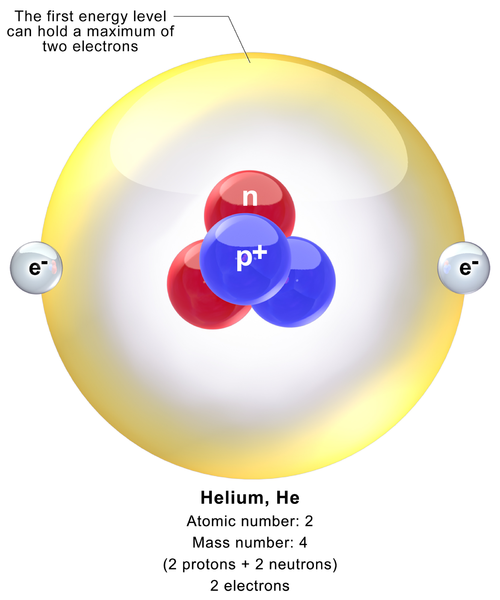
Difference Between Helium and Hydrogen Properties, Isotopes, Reactions, Applications
Li = 75:62eV for helium and lithium, respec-tively. For the ionization of the third lithium electron an energy of I(3) Li = 122:42eV is required. For full thermal ionization of all helium or lithium electrons we need far more than 100eV, i.e. more than 106 K. An alternative way to reach high ionization degrees is to increase the particle Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent.




