Download eSaral App. Get detailed study material for Class 11 Chemistry to prepare for Boards as well as competitive exams like IIT JEE, NEET etc. eSaral offers you complete notes of Isomerism Class 11th. eSaral helps the students in clearing and understanding each topic in a better way. eSaral is providing complete chapter notes of Class 11th. When the isomers have similar molecular formula but differ in nature of carbon chain are called chain isomers and phenomenon is known as chain isomerism. Example: C 4 H 10. 11 N C 2 H 5 - NH - C 2 H 5: Diethyl amine C 3 H 7 - NH - CH 3: Methyl propyl amine (e) TAUTOMERISM ( Greek word : tauto = same ; meros = parts)
Isomerism Notes for Class 11, IIT JEE & NEET
This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion. One example of ionisation isomerism is [Co (NH 3) 5 SO 4 ]Br and [Co (NH 3) 5 Br]SO 4. We can prepare these ionisation isomers in the following method. Notes of Class11(A&B) Alok Sir, Chemistry isomerism.pdf - Study Material. Notes of Class11(A&B) Alok Sir, Chemistry isomerism.pdf - Study Material. Dashboard Login. class-11th. Chemistry. 0 Likes. 28 Views. Copied to clipboard A. Alok Ranjan Dhungia. Jan 21, 2022. Study Material. Class XI and Class XII NCERT can really test your understanding of fundamental of Chemistry. In fact this book excellently covers all types of numericals related to Organic Chemistry.. Chemistry, Isomerism Notes, Solved Examples. Get Notes prepared by Ex-IITIan for IIT JEE (Main) Chemistry , Isomerism. O P Tondon IIT JEE (Main) Chemistry. There are three types of structural isomerism •Chain isomerism •Position isomerism •Functional group isomerism Chain isomerism: Compounds with the same molecular formula but different structures of the carbon skeleton These isomers arise because of the carbon chains can be branched. For example, there are two isomers of butane, C4H10. In.

isomerism class 11 Isomerism L1 Chain, Position Isomer Organic Chemistry NEET & JEE
NCERT Isomerism Class 11 Handwritten Notes | PDF. isomerism class 11 handwritten notes - Read online for free. Optical isomers do not exhibit symmetry and do not have identical mirror images. It is a type of isomerism in which molecules have the same molecular and structural formulae, but are non-super imposable mirror images of each other. An example is butan-2-ol. It has four different groups attached to its second carbon atom. Structural isomerism The functional groups and the atoms in the molecules of these isomers are linked in different ways. Different structural isomers are assigned different IUPAC names since they may or may not contain the same functional group. The different types of structural isomerism are discussed below. 1.Chain Isomerism 2. Position Isomerism 3. Functional.
Isomerism NEET Previous Year Questions with Complete Solutions
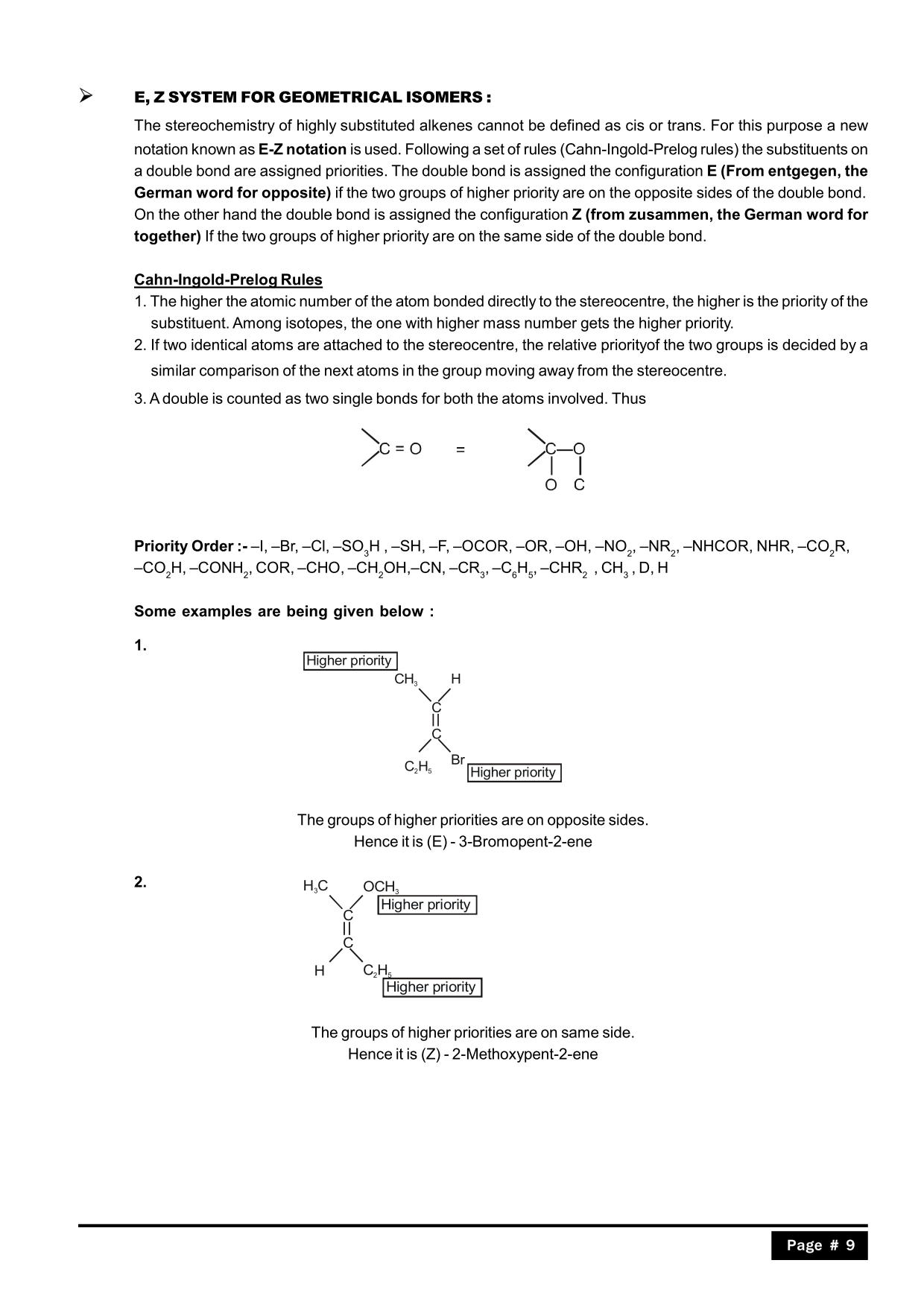
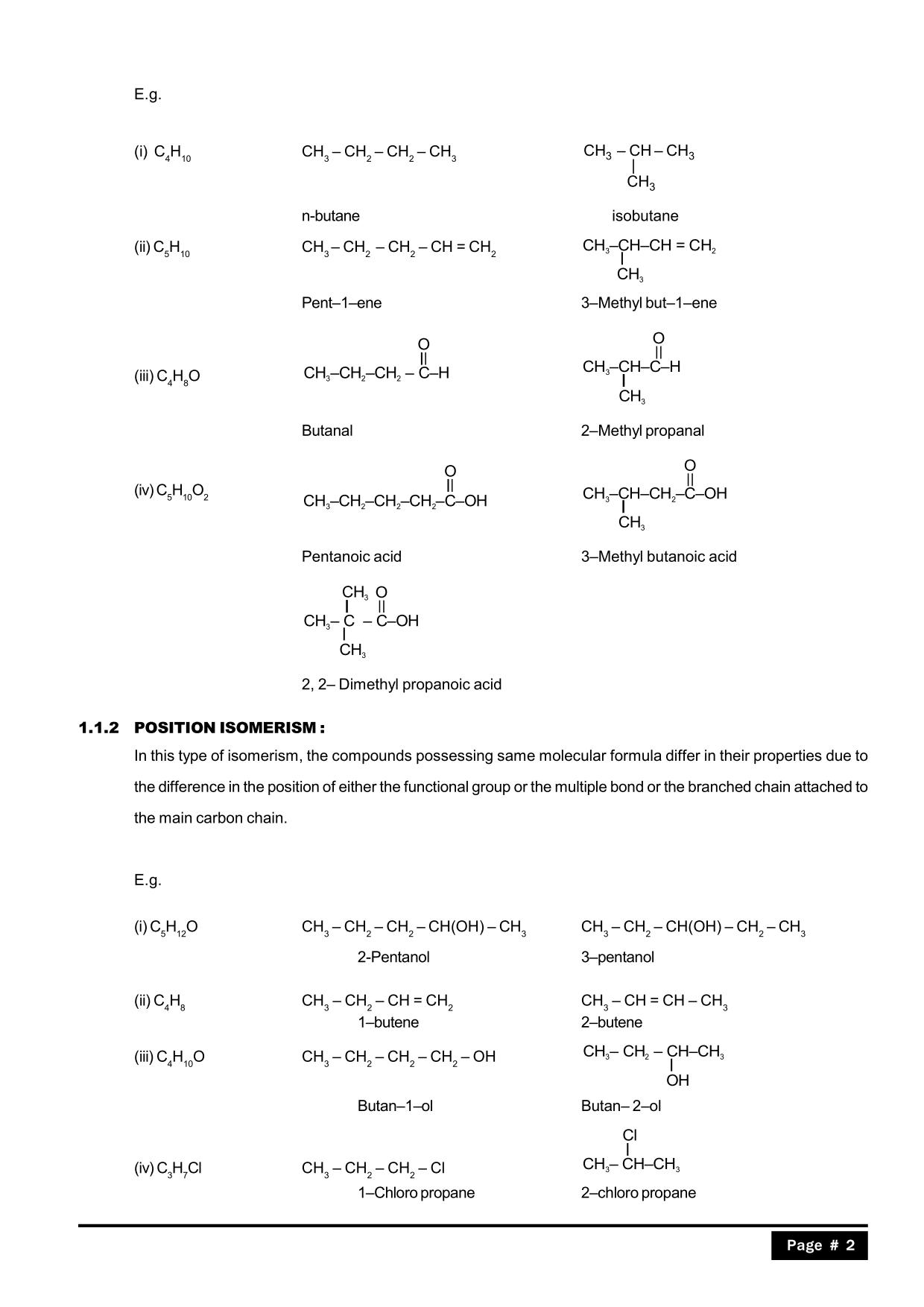
Full syllabus notes, lecture and questions for Geometrical Isomerism - Chemistry Class 11 - NEET - NEET - Plus excerises question with solution to help you revise complete syllabus for Chemistry Class 11 - Best notes, free PDF download Compounds which have the same molecular formula but different structural formulas are called isomers and the phenomenon is known as isomerism. Type of Structural Isomerism: (a) Chain Isomerism: Chain isomers possess the same molecular formula, but different number of carbon in parent-chains (straight or branched). (i) Butane: C 4 H 10
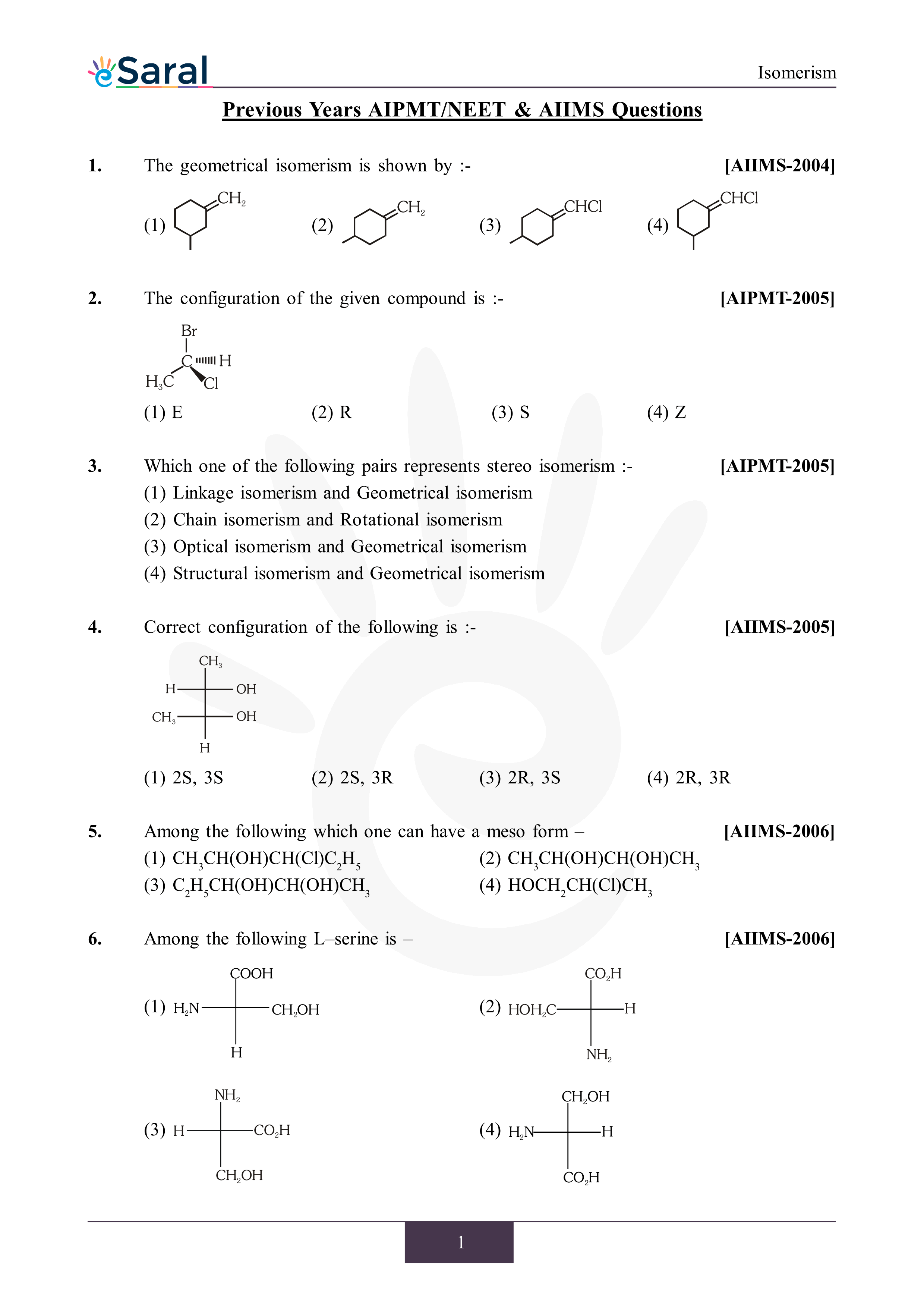
Learn about Isomerism topic of Chemistry in details explained by subject experts on vedantu.com. Register free for online tutoring session to clear your doubts.. Download PDF. NCERT Solutions. NCERT Solutions for Class 12. NCERT Solutions for Class 11.. CBSE Notes for class 11. CBSE Notes for class 10. CBSE Notes for class 9. CBSE Notes. Document Description: Isomerism in Alkanes, Alkenes & Alkynes for NEET 2023 is part of Chemistry Class 11 preparation. The notes and questions for Isomerism in Alkanes, Alkenes & Alkynes have been prepared according to the NEET exam syllabus. Information about Isomerism in Alkanes, Alkenes & Alkynes covers topics like and Isomerism in Alkanes.
Isomerism Notes for Class 11, IIT JEE & NEET
Document Description: Structural Isomerism for NEET 2024 is part of Chemistry Class 11 preparation. The notes and questions for Structural Isomerism have been prepared according to the NEET exam syllabus. Information about Structural Isomerism covers topics like Classification:, Structural isomerism:, Chain Isomerism:, Position Isomerism. Class 11 Syllabus; Class 12 Syllabus; Sandeep Garg Solutions. Sandeep Garg Class 11 Economics; Sandeep Garg Class 12 Economics; TS Grewal Solutions. TS Grewal Class 11 Accountancy; TS Grewal Class 12 Accountancy; Sample Papers Class 11. Class 11 Business Studies; Class 11 Economics; Entrance Exams. COMED-K. Previous Year Question Papers; Sample.




