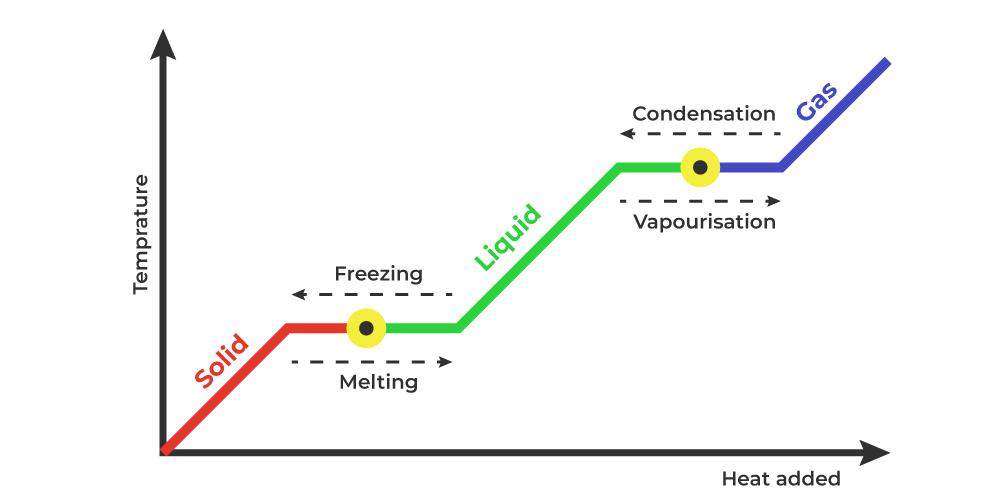
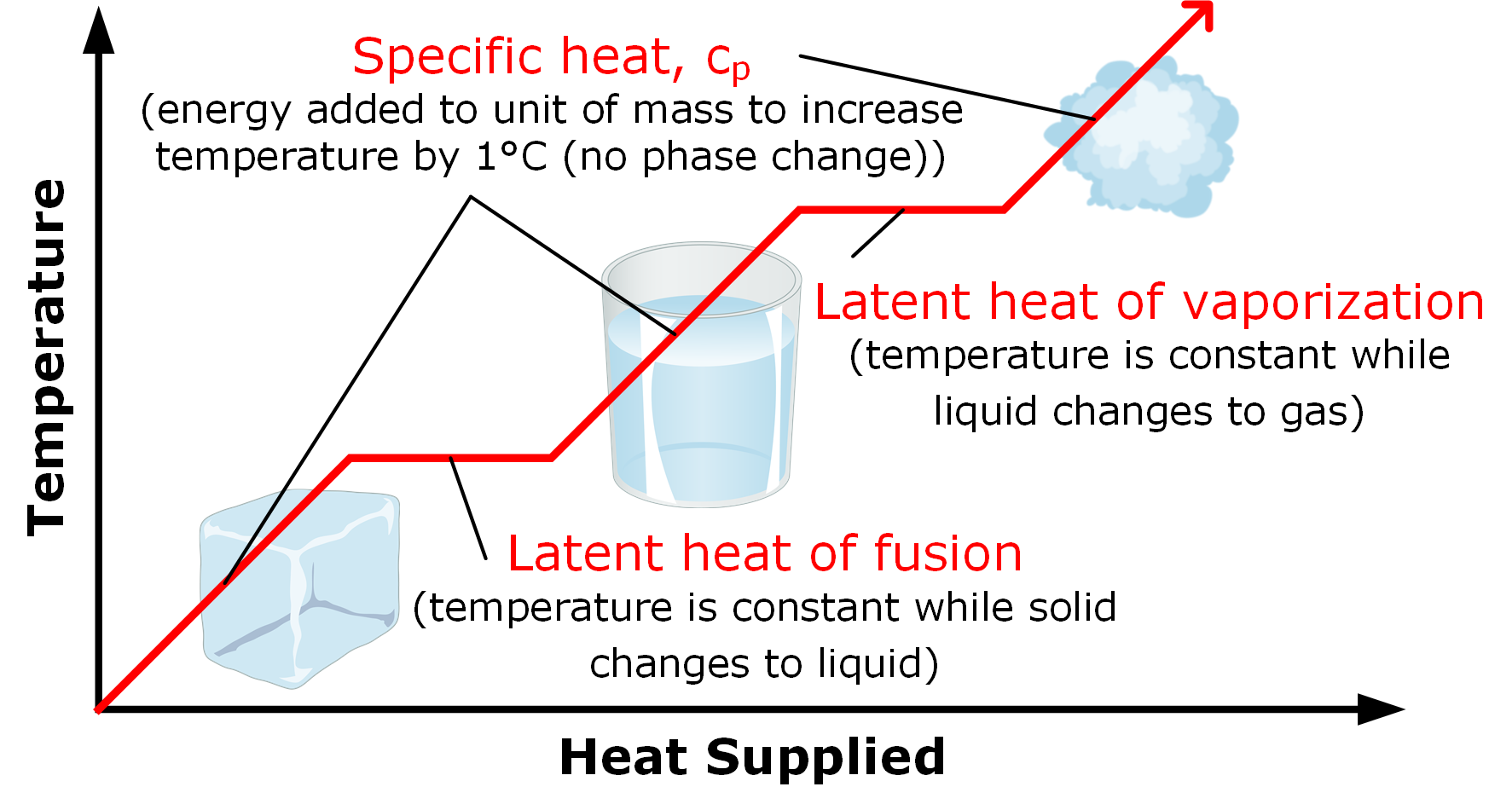
Because this energy enters or leaves a system during a phase change without causing a temperature change in the system, it is known as latent heat (latent means hidden ). The three phases of matter that you frequently encounter are solid, liquid and gas (see Figure 11.8 ). Phase changes Transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the specific heat.If heat were added at a constant rate to a mass of ice to take it through its phase changes to liquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization) would.

Suggest a method to liquefy atmospheric gases Teachoo Science
The latent heat of fusion refers to the phase change between states of solid and liquid. Here, heat actually refers to the transfer of heat energy between the objects. Thus, the latent heat of fusion encompasses the process of adding heat to melt some solid. The formula for Latent heat of fusion: Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically in the form of heat) in order to trigger a change in its physical state and convert it into a liquid (when the pressure of the environment is kept constant). Using the equation for a change in temperature and the value for water from Table 14.2, we find that Q = mLf =(1.0kg)(334kJ/kg)= 334 kJ Q = mL f = ( 1. 0 kg) ( 334 kJ/kg) = 334 kJ is the energy to melt a kilogram of ice. This is a lot of energy as it represents the same amount of energy needed to raise the temperature of 1 kg of liquid water. construct a phase diagram by specifying the phase(s) observed at particular P,T combinations: t. the latent heat involves a change in internal energy, plus work in expanding the volume. Examples of latent heats: material fusion, L f (J / kg) vaporization, L v (J / kg) water 33.5 x 104 22.6 x 105 ethyl alcohol 10.8 x 104 8.55 x 105

Measuring the specific latent heat of fusion of Ice YouTube
Overview. The 'enthalpy' of fusion is a latent heat, because, while melting, the heat energy needed to change the substance from solid to liquid at atmospheric pressure is latent heat of fusion, as the temperature remains constant during the process.The latent heat of fusion is the enthalpy change of any amount of substance when it melts. When the heat of fusion is referenced to a unit of mass. 18.7: Latent Heat of Fusion. The most straightforward method for measuring the specific latent heat L of ice is to drop a lump of. Ice of mass m and specific latent heat L at its melting point T0 into a. Calorimeter of mass MC and specific heat capacity CC and initial (warm) temperature T2, a mass MW of Water of specific heat capacity CW at the. Latent heat is measured in units of J/kg. Both L f and L v depend on the substance, particularly on the strength of its molecular forces as noted earlier. L f and L v are collectively called latent heat coefficients.They are latent, or hidden, because in phase changes, energy enters or leaves a system without causing a temperature change in the system; so, in effect, the energy is hidden. Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation.. Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or.
Latent Heat of Fusion Definition, Formula, Examples & FAQs
I explain the definition of Latent Heat and how it's used in the equation Q = mL, how to identify the latent heat of fusion, vaporization, and sublimation, a. What the heck is dry ice and why is it so spooky? Learn this and more when we investigate phase changes and phase diagrams!Watch the whole General Chemistry.
Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. It could either be from a gas to a liquid or liquid to a solid and vice versa. Latent heat is related to a heat property called enthalpy. Download Complete Chapter Notes of Thermodynamics Download Now Phase Diagrams; What is Heat of Fusion? Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. When studying chemistry, "fusion" simply has the same definition as melting. In the classroom, you mostly use heat of fusion when.
Phase Change Requires Heat FD2021 Fundamentals of Fire and Combustion on Guides
Step 3: Calculate the latent heat of fusion. Calculate the specific latent heat of fusion of ice to water using the equation ΔE = LΔm. ΔE = Energy supplied by the heater = 10, 000 J; L = Specific latent heat of fusion; Δm = mass of ice; Rearrange the equation; So, = 340136 J kg-1 = 340 000 J In Chemistry, Latent heat is heat that occurs when a substance changes physically without changing its temperature as a result of the release or absorption of energy. Ice, Water have different latent heat of fusion. Not only this but it is also having a specific latent heat of fusion.




