1. Write down the electronic configuration of N2 atoms N 2 is composed of two nitrogen (N) atoms. The electronic configuration of each N-atom is 1s2 2s2 2px1 2py1 2pz1. Usually, only the valence electrons are displayed in the MO diagram of a molecule, therefore, it is important to note that each N-atom contains 5 valence electrons. Molecular Orbitals for N2 Jmol models of calculated wavefunctions To view a model, click in the circle of a molecular orbital in the energy level correlation diagram shown Ignore any popup warning and click on the green Continue button which appears Mouse Control of Models

a) Simplified N2 orbital diagram. Reproduced with permission.104... Download Scientific Diagram
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start. Lewis Dot Structure The Lewis structure indicates the atom and its position in the model of the molecule using its chemical symbol. It also describes the chemical bonding between atoms present in the molecule. Mainly, the structure depicts the arrangement of the valence shell electrons of an element. This video explains easy way of drawing Molecular orbital diagram of N2 molecule.Nitrogen molecule is formed by the combination of two N-atoms. When they com. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory..

12+ N2 Molecular Orbital Diagram Robhosking Diagram
26 I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For NX2 the orbitals in increasing energy are: σ1s <σ∗1s <σ2s <σ∗2s <π2px,π2py <σ2pz < π∗2px,π∗2py <σ∗2pz because it has 14 electrons. For NX2X− there are 15 electrons. Remember: When two Nitrogen atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.N2(-) has a bond order. STEP 3. Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. Keep in mind the energy of the atomic orbitals and molecular orbitals! The following factors contribute to the position of one MO with respect to other MOs. More nodes = more energetic = higher MOs. Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory (MOT) and linear combination of atomic orbital (LCAO). The molecular orbital diagram has molecular orbital energy level at centre and is surrounded by atomic orbital energy level.
N2 Molecular Orbital Diagram Free Diagram For Student
If we build the MO diagram for N2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. Orbital Overlap Diagram for N2 chemistNATE 247K subscribers Subscribe 318 16K views 2 years ago Here, we draw the orbitals that overlap to hold together a molecule of N2, nitrogen gas,.
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.. Between [latex]\ce{N2}[/latex] and [latex]\ce{O2}[/latex], the order of the. A molecule must have as many molecular orbitals as there are atomic orbitals. Figure 9.7.1 9.7. 1: Molecular Orbitals for the H 2 Molecule. (a) This diagram shows the formation of a bonding σ 1s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1 s atomic orbitals.

(a) N atom orbitals and their linear combination to form N2 molecular... Download Scientific
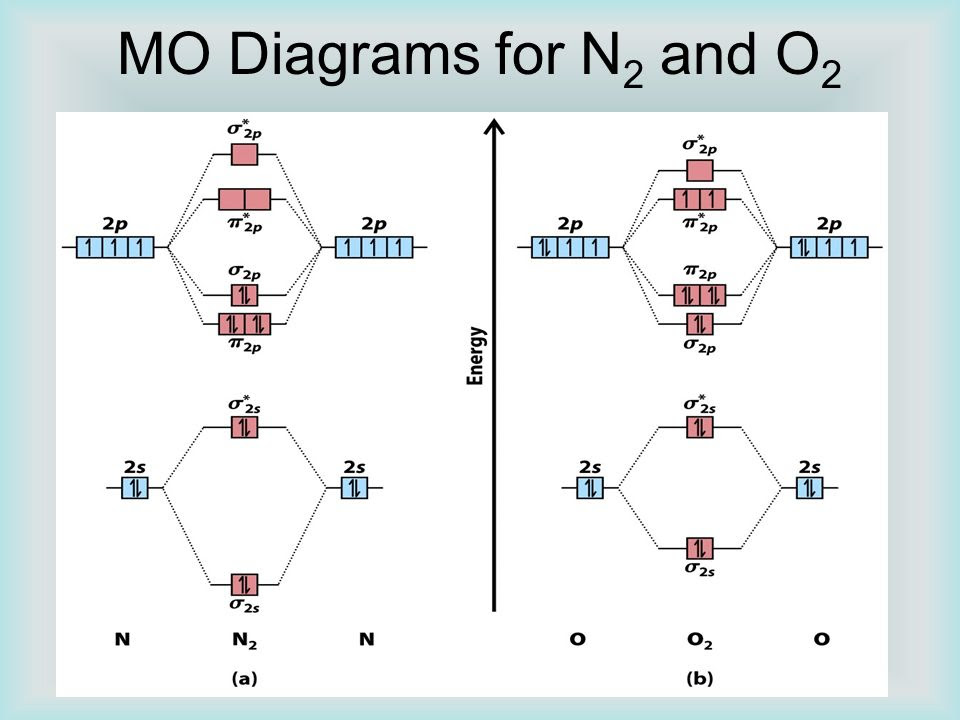
Figure 9.8.4: Molecular Orbital Energy-Level Diagram for a Heteronuclear Diatomic Molecule AB, Where χ B > χ A. The bonding molecular orbitals are closer in energy to the atomic orbitals of the more electronegative B atom. Consequently, the electrons in the bonding orbitals are not shared equally between the two atoms. The molecular orbital diagram for N2 shows that there are two electrons in the sigma bonding molecular orbital, which results in a stable double bond between the nitrogen atoms. The sigma antibonding orbital remains unoccupied. This diagram indicates that N2 has a triple bond with a strong bond strength due to the stable sigma bonding molecular.


