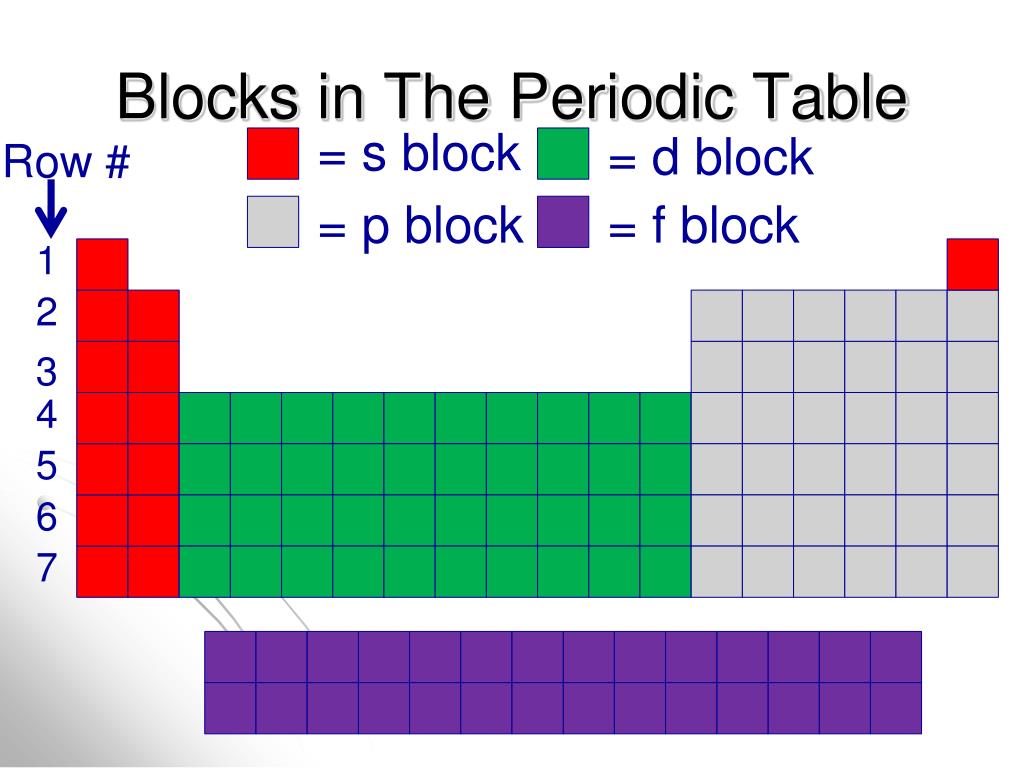
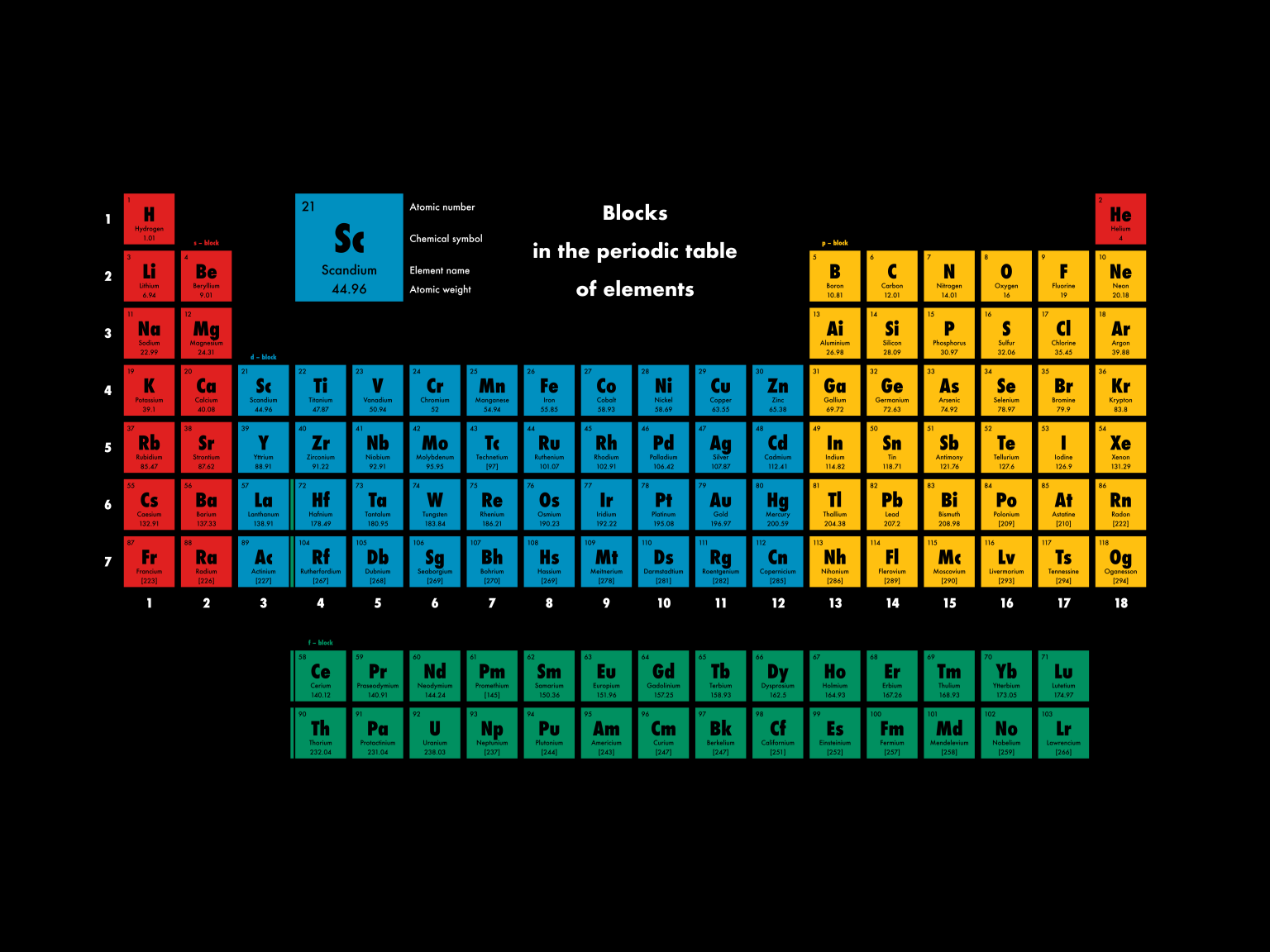
Block (periodic table) Blocks s, f, d, and p in the periodic table Part of a series on the Periodic table Periodic table forms Periodic table history Sets of elements By periodic table structure Groups (1-18) 1 ( alkali metals) 2 (alkaline earth metals) 3 4 5 6 7 8 9 10 11 12 13 14 15 (pnictogens) 16 (chalcogens) 17 (halogens) 18 (noble gases) An element block is a set of elements located in adjacent element groups. Charles Janet first applied the term (in French). The block names (s, p, d, f) originated from descriptions of spectroscopic lines of atomic orbitals: sharp, principal, diffuse, and fundamental.
PPT Blocks in The Periodic Table PowerPoint Presentation, free download ID2061175
Periodic table blocks are sets of elements grouped by their valence electron orbitals. The four block names are s-block, p-block, d-block, and f-block. Should a new element be discovered, it will be in g-block. Each block indicates which electron sublevel is in the process of being filled. The length of each period is determined by the number of electrons that are capable of occupying the sublevels that fill during that period, as seen in the table below. Period. Number of Elements in Period. Sublevels in Order of Fill. Table 6.8.1 6.8. 1: Period Length and Sublevels in the Periodic Table. Classification of elements into groups The six noble gases—helium, neon, argon, krypton, xenon, and radon—occur at the ends of the six completed periods and constitute the Group 18 (0) group of the periodic system. It is customary to refer to horizontal series of elements in the table as periods and vertical series as groups. About Transcript The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity. Created by Sal Khan. Questions Tips & Thanks
Blocks in the periodic table of elements by Tony Gale on Dribbble
Sn is located in the second column of the p block, so we expect that its electron configuration would end in p 2. Tin's electron configuration is [Kr]5 s 2 4 d 10 5 p 2. From the element's position on the periodic table, predict the valence shell electron configuration for each atom. Figure 14.3.11 14.3. Group 14 is the 4 th column in the main group, or "A-Block," columns of the periodic table and so is labeled as Group 4A. Cd (cadmium) is located in the 12 th column of the periodic table. In the "1-18 System," this column is labeled as Group 12. Bromine (Br) is located in the 17 th column of the periodic table. A block on the periodic table is a group of elements that all have their electrons in the same atomic orbital. There are four blocks, s-, d-, f, and p-. [1] The word "block" was first used to describe this by Charles Janet. [2] Blocks p-block The p-block is on the right side of the periodic table. Elements from groups 13-18 are in the p-block. Periodic Table of Elements TABLE LIST W/PROPERTIES GAME Display Property/Trend 17 Cl Chlorine halogen Plot Atomic Mass 1 H Hydrogen nonmetal 2 He Helium noble gas 3 Li Lithium alkali metal 4 Be Beryllium alkaline earth metal 5 B Boron metalloid 6 C Carbon nonmetal 7 N Nitrogen nonmetal 8

periodic table of the elements paperzip periodic table of elements hd KristinaxyCouch75b
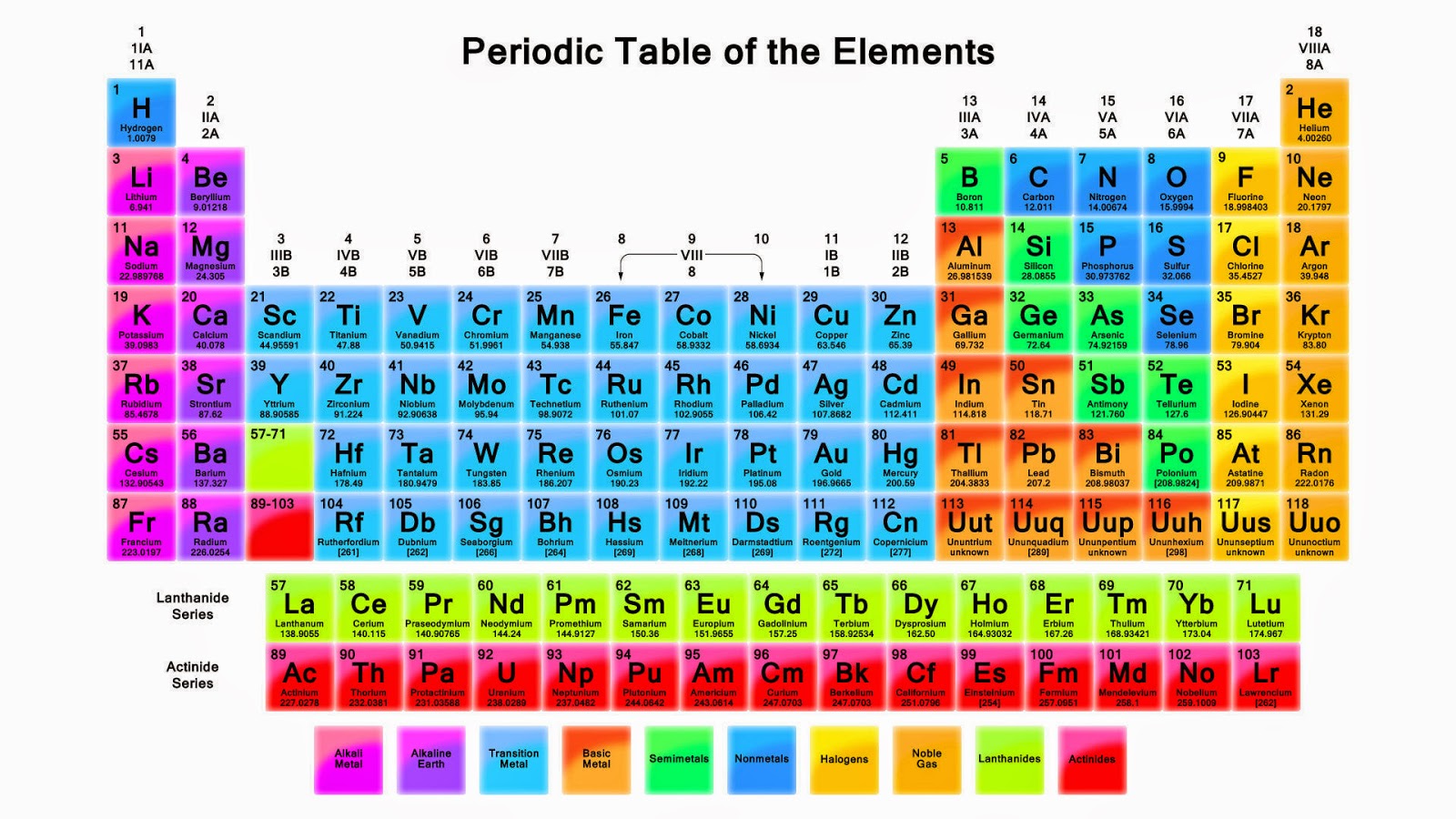
Category Chemistry Portal v t e The periodic table, also known as the periodic table of the elements, arranges the chemical elements into rows ("periods") and columns ("groups"). It is an icon of chemistry and is widely used in physics and other sciences. Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
The electron configurations of the elements are in Figure 6.9.2. Because each orbital can have a maximum of 2 electrons, there are 2 columns in the s block, 6 columns in the p block, 10 columns in the d block, and 14 columns in the f block. Hydrogen and helium are placed somewhat arbitrarily. Although hydrogen is not an alkali metal, its 1 s1. HowStuffWorks. Each block of the periodic table houses an element, along with a few standard facts about that element: Atomic number: integer equal to the number of protons or electrons in the element. Gold's atomic number is 79. Element symbol: one or two letters. In the case of two letters, the first one is always capitalized.
Everything's Here sblock Elements and pblock Elements
The periodic table of the elements, shown in Figure 2.4, is a chart identifying the 92 elements found in nature, as well as several larger, unstable elements discovered experimentally. The elements are arranged in order of their atomic number, with hydrogen and helium at the top of the table, and the more massive elements below. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term seems to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, f-block and g-block. Blocks s, f, d, and p in the periodic table




