Changing by Heating or Cooling Heat is commonly used to change substances. Some of these changes can be reversed by cooling, but others are irreversible. This depends on the properties of the substance that is being heated and cooled. Reversible Changes When water is heated and cooled it goes through a series of reversible changes. Reversible and Irreversible Changes- Science Animation Class 6th The changes taking place can be categorized into two parts: (a) Reversible Change Show more Show more Introduction to Chemical.

Reversible & Irreversible Teaching chemistry, Chemical and physical changes, Physical change
A change which can happen backward, that is, can be reversed is called a reversible change. If you keep water in the freezer for some time, it transforms into ice. But as soon as you take it out of the freezer, it turns into water again. This is a reversible change. Similarly, if you boil water, it evaporates and becomes water vapor. A reversible change changes how a substance looks or feels (changing the physical appearance), and it is easy to turn it back again. A reversible change may change the state of a substance such as, solid, liquid and gas. However, a reversible change does not change the amount of matter in a substance. Reversible change Evaporation is taking place: a liquid is turning into a gas. Condensation is taking place: a gas is turning into a liquid. Both evaporation and condensation are taking place. These labels are for sticking onto a Venn diagram for sorting the cards. http://www.collaborativelearning.org/reversiblechange.pdf Aasoka presents a video that aids in the understanding of the continuous change that all the matter around us undergo. It uses engaging animations to explain.
Reversible Changes and Irreversible Changes Learn Important Terms and Concepts
A reversible change might change how a material looks or feels. It sometimes creates new materials. Image caption, Freezing is a reversible change. For example you can freeze juice to make ice. A reversible change is a chemical change where no new materials are created, and the original material can be recovered. Examples include freezing water to make ice or melting chocolate. What's the difference between a reversible change and an irreversible change? Examples of Physical Changes. Remember, the appearance of matter changes in a physical change, but its chemical identity remains the same. Crushing a can. Melting an ice cube. Boiling water. Mixing sand and water. Breaking a glass. Dissolving sugar and water. Shredding paper. A reversible change is a chemical change where no new materials are created and the original material can be recovered. Examples include freezing water to make ice or melting chocolate. What's the difference between a reversible change and an irreversible change?

PPT Types of Changes PowerPoint Presentation, free download ID2730493
As the name suggests, reversible changes are those changes whose effect can be reversed. Reversible means something that can be traced back to its original form. This article discusses about different types of changes that take place. Then we will discuss about reversible change in particular and then see different examples of reversible change. 9,240 reversible change stock photos and photography are available royalty-free. See reversible change stock video clips Image type Orientation Color People Artists AI Generated More Sort by Popular Money and Financial Concepts Car Types Jobs/Professions Motor car Water filter Reverse osmosis Plumber Coin Gear stick Automatic transmission of 93
CBSE Notes Introduction to Reversible and Irreversible Changes Changes are the only constant thing around us. Changes can happen to both living and non-living things. The changes that happen around us can sometimes be reversible and sometimes irreversible. Reversible changes or physical changes are changes that can be undone or reversed. Melting, freezing, boiling, evaporating, condensing, dissolving and also,.
Reversible And Irreversible Changes
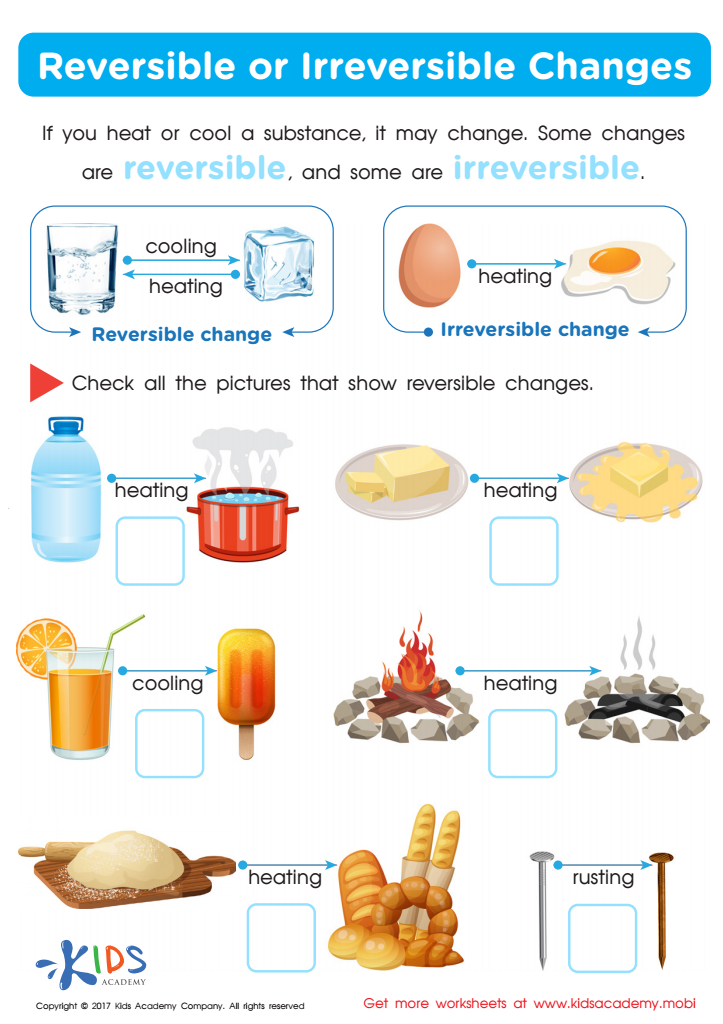
This worksheet forms part of lesson titled, 'What does reversible and irreversible change mean', which covers the following science topics: Graphs and pictures explaining the difference between temporary and permanent transformations; Chemical reactions (when sugar crystals dissolve), reversible reactions (when chocolate melts), and. Reversible Changes Any changes which can be reversed or are a temporary conversion are known as reversible changes. The reactions which are reversible are called reversible reactions. In this reaction, one substance is modified into another form but a new compound is not formed.




