A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (Nitrate ion).For the NO3- structure use the periodic table to find the total number o. Lewis Dot Structure of NO3- (Nitrate Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 294K views 12 years ago Every Video I quickly take you through how to draw the Lewis.

what is resonance?resonating structure of NO3 ion Brainly.in
This chemistry video tutorial explains how to draw the lewis structure of the nitrate ion NO3-.Chemistry - Basic Introduction: https://www. How to Draw the Lewis Dot Structure for NO3 - (Nitrate ion) - YouTube A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (the Nitrate ion).For the NO3 - structure. Construction of NO3 Lewis Dot Structure 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. Following steps are required to draw NO 3- lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of nitrogen and oxygen atoms and including charge of the anion Total electrons pairs in valence shells Center atom selection from nitrogen and oxygen atom Put lone pairs on atoms

NO3 Lewis Structure, Molecular Geometry, and Hybridization
Lewis structure of NO3- ion (nitrate ion) contains one double bond and two single bonds between the Nitrogen (N) atom and Oxygen (O) atoms. The Nitrogen atom (N) is at the center and it is surrounded by 3 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. There are 24 valence electrons available for the Lewis structure for NO 3-. Video: Drawing the Lewis Structure for NO3- n It is helpful if you: Try to draw the NO 3- Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

NO3 Molecular Geometry / Shape and Bond Angles YouTube
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. A simple procedure for writing Lewis Dot Structures is shown in this video.Several worked examples relevant to this procedure are given.http://chem-net.blogs.
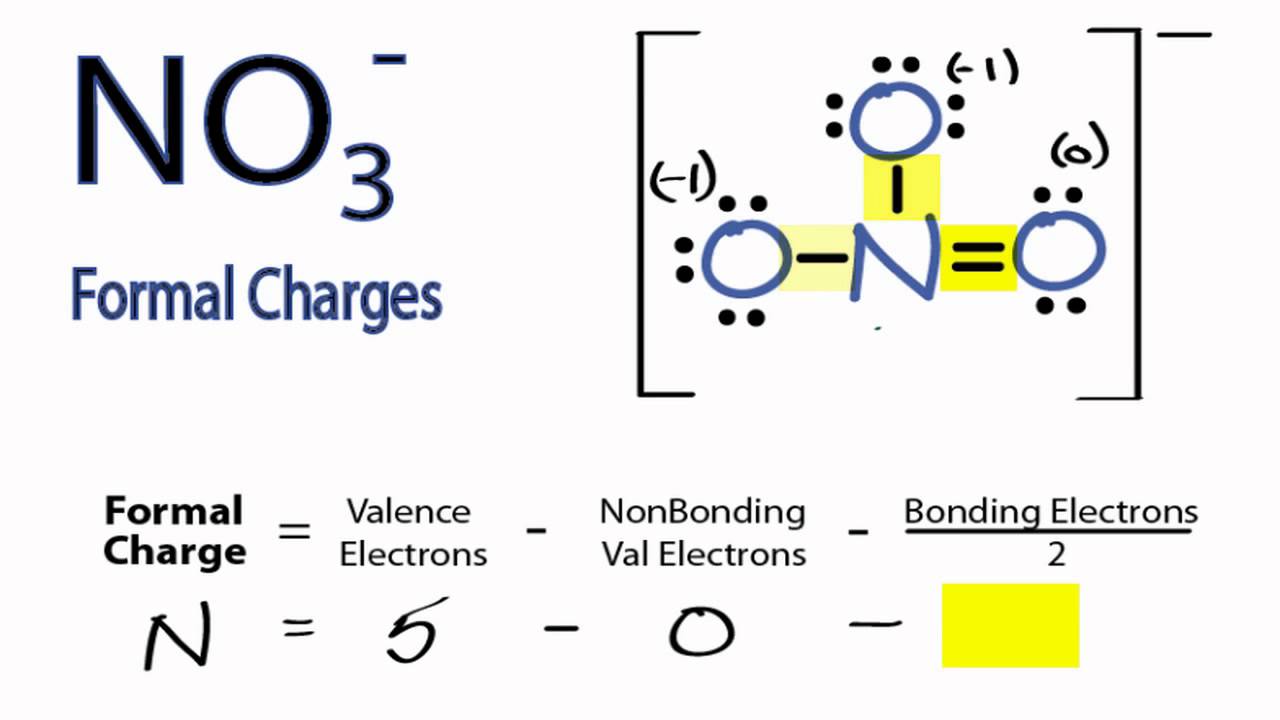
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.". Transcript: This is the NO3- Lewis structure: the nitrate ion. Nitrogen has 5 valence electrons. Oxygen has 6, we have 3 Oxygens, and we need to add 1 for this valence electron up here. That gives us a total of 5 plus 18 plus 1: 24 valence electrons. Nitrogen is the least electronegative; we'll put that in the center, and we'll put the Oxygens.
no2 bond order
A dot structure is any representation of atoms/molecules using dots for electrons. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. There's a slight difference, but they effectively mean the same thing. GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. Dot • one dot represents one valence electron (found on odd-electron particles). 2. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and "belong to" only one atom. 3. Dash each dash represents two electrons that are shared between two atoms as a covalent bond.




