How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check. The lewis structure is also called an electron dot structure which determines the number of valence electrons present in an atom. Moreover, they also describe how these valence electrons are participating in the bond formation to form a molecule.
SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur
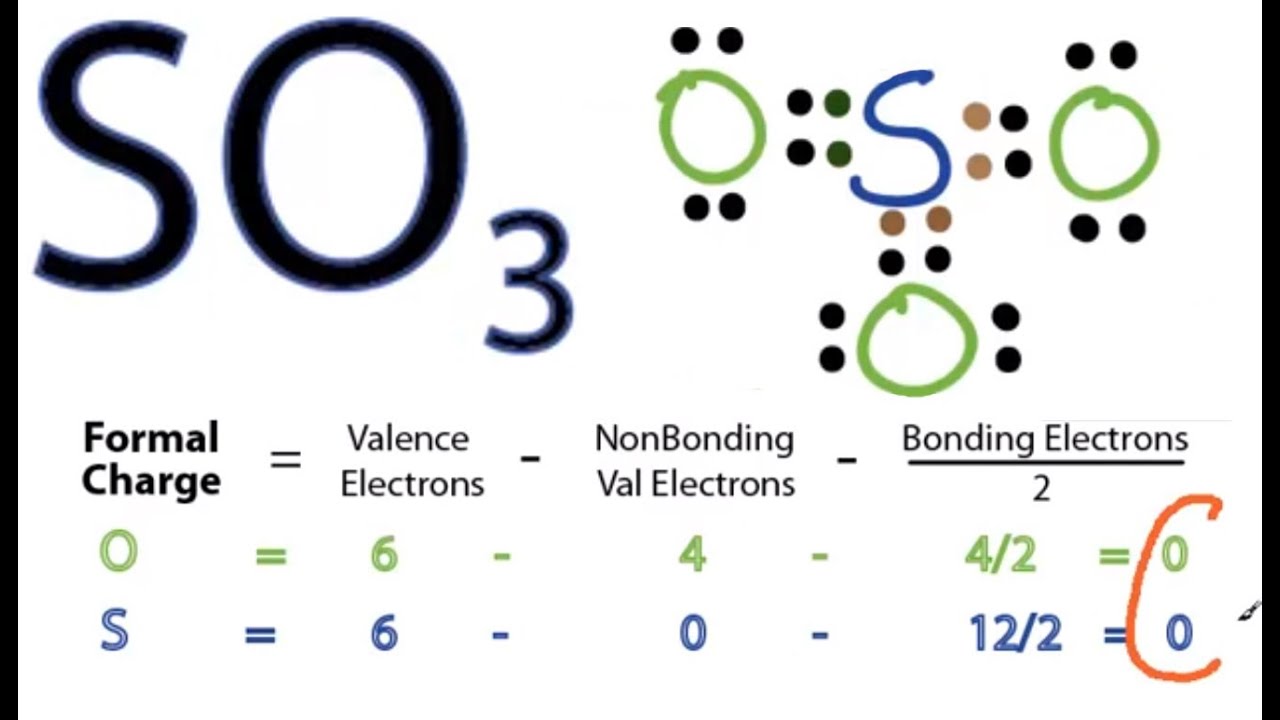
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number. This chemistry video explains how to draw the Lewis structure of SO3 - Sulfur Trioxide. It discusses the molecular geometry, bond angle, hybridization, and. Lewis structure of SO3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair while all the three Oxygen atoms have 2 lone pairs. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.

Lewis Dot Diagram For So3 Wiring Diagram
Website-http://www.kentchemistry.com/links/bonding/LewisDotTutorials/SO3.htmI quickly take you through how to draw the Lewis Structure of SO3 (Sulfur Trioxid. The SO3 Lewis structure illustrates how the atoms of sulfur trioxide, a molecule composed of one sulfur atom and three oxygen atoms, are arranged. Within the SO 3 Lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. Additionally, each oxygen atom has two lone pairs of electrons associated with it. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 . Video: Drawing the Lewis Structure for SO3 It is helpful if you: Try to draw the SO 3 Lewis structure before watching the video. Lewis structure of SO 3 molecule There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Each oxygen atom has two lone pairs in SO 3 lewis structure. But, there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2. Hybridization of SO 3 molecule All atoms have sp 2 hybridization.

SO3 Lewis Structure, Molecular Geometry, and Hybridization
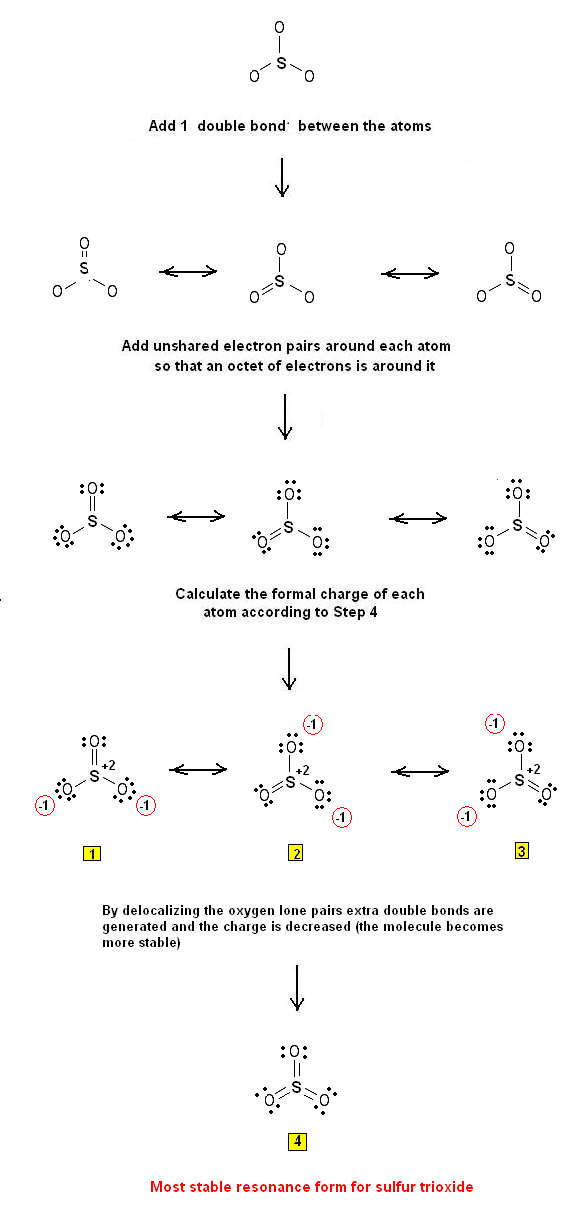
SO3, known as sulphur trioxide is sp2 hybridized with a triagonal planar structure and having bond angle 1200. It is a colourless or white crystalline solid with boiling and melting point 450C and 16.90C respectively. It is a covalent compound having total three double bonds in between sulphur and oxygen are present in SO3 structure. Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
So 6 minus zero minus 12 over 2; so 6 minus 6 equals zero. So we can write the formal charge for Sulfur as zero. So we have formal charges of zero for each of the atoms in SO3. That makes this the best Lewis structure for SO3. This is Dr. B., and thanks for watching. The first step is to sketch the Lewis structure of the SO3 molecule, to add valence electrons around the sulfur atom; the second step is to add valence electrons to the three oxygen atoms, and the final step is to combine the step1 and step2 to get the SO3 Lewis Structure.
Simple Procedure for writing Lewis Structures Lewis Structures for
Sulfur brings 6, and oxygen brings 3 each. That means; SO3 has 24 valence electrons. 6 + (3 x 6) = 24. Now have a look of Lewis Structure again; When we draw it, firstly we get the three structures at the top. Sulfur in the center and Oxygen around it is making a connection (each) to the central atom. There should be single bonds initially. SO3 lewis structure has a Sulfur atom (S) at the center which is surrounded by three Oxygen atoms (O). There are 3 double bonds between the Sulfur atom (S) and each Oxygen atom (O).. In the above lewis dot structure of SO3, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following.


