An enthalpy-entropy chart, also known as the H-S chart or Mollier diagram, plots the total heat against entropy, [1] describing the enthalpy of a thermodynamic system. [2] A typical chart covers a pressure range of .01-1000 bar, and temperatures up to 800 degrees Celsius. [3] Since enthalpy is a state function, a change in enthalpy does not depend on the pathway between two states. Hess's law: In going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps.
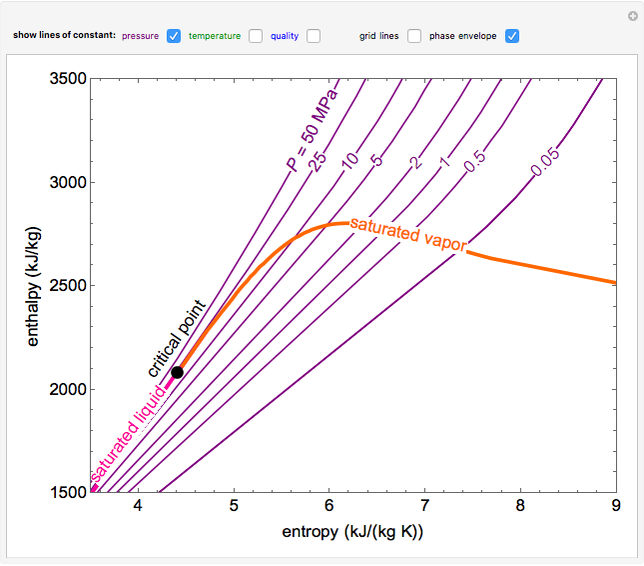
enthalpyentropydiagramforwater LearnChemE
25°C, 1 atm Properties of some common fuels and hydrocarbons Natural logarithms of the equilibrium constant Kp Generalized enthalpy departure chart Generalized entropy departure chart Psychrometric chart at 1 atm total pressure One-dimensional isentropic compressible-flow functions for an ideal gas with k 1.4 One-dimensional normal-shock functio. 34 Formula State of Matter Enthalpy (kJ/mol) Entropy (J mol/K) Gibbs Free Energy (kJ/mol) Ba 2TiO 4 (s) -2243.0424 196.648 -2133.0032 BaBr 2 (s) -757.304 146.44 -736.8024 BaBr 2 (g) -439.32 330.536 -472.792 BaBr 2•2H 2O (s) -1366.076 225.936 -1230.5144 BaCl 2 (s) -858.1384 123.67904 -810.4408 BaCl 2 (l) -832.44864 143.5112 -790.1484 BaCl 2 (g) -498.7328 325.64072 -510.69904 The Mollier diagram, also called the enthalpy (h) - entropy (s) chart or h-s chart, is a graphical representation of thermodynamic properties of materials. In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. Mollier Diagram Origins Standard Thermodynamic Properties for Selected Substances. 88. 162. As an Amazon Associate we earn from qualifying purchases. This free textbook is an OpenStax resource written to increase student access to high-quality, peer-reviewed learning materials.

Nitrogen Enthalpy, Internal Energy and Entropy vs. Temperature
Table of Thermodynamic Values - University of Wisconsin-Madison An enthalpy-entropy chart, also known as the H-S chart or Mollier diagram, plots the total heat against entropy, describing the enthalpy of a thermodynamic system. A typical chart covers a pressure range of .01-1000 bar, and temperatures up to 800 degrees Celsius. It shows enthalpy in terms of internal energy , pressure and volume using. The disordered enthalpy-entropy descriptor is a mathematical formula that accelerates the computational discovery of synthesizable high-entropy ceramics, and has already guided the synthesis of. Of course, the main issue here is how entropy changes during a process. This can be determined by calculation from standard entropy values (\ (S^ o\)) in the same way that enthalpy changes are calculated: ∑So products − ∑So reactants = ΔSorxn (6.5.2) (6.5.2) ∑ S p r o d u c t s o − ∑ S r e a c t a n t s o = Δ S r x n o.
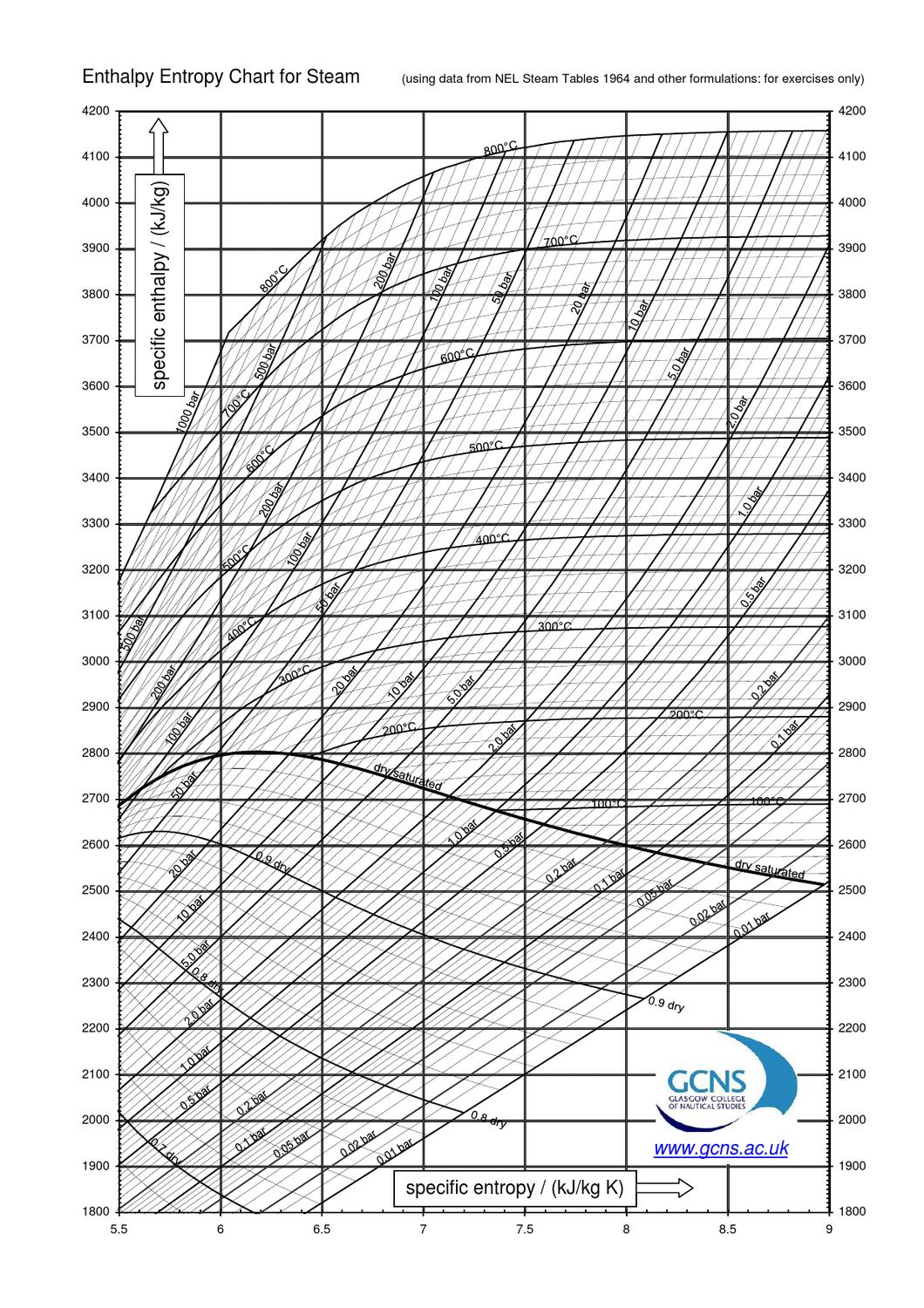
Enthalpy Entropy Chart for Steam by Sandy Small Issuu
Miscellaneous Physiology Piping Systems Sanitary Drainage Systems Standard Organizations Statics Steam and Condensate Thermodynamics Water Systems Enthalpy-entropy diagram for water and steam. An enthalpy-entropy chart, also known as the H-S chart or Mollier diagram, plots the total heat against entropy, describing the enthalpy of a thermodynamic system. A typical chart covers a pressure range of .01-1000 bar, and temperatures up to 800 degrees Celsius.
Mollier's H-S diagram (Enthalpy v Entropy) was a logical extension of the T-S diagram (Temperature v Entropy) first proposed by Gibbs, retaining the advantages of T-S diagrams but introducing several new advantages. A typical H-S Mollier diagram for a thermodynamic fluid such as steam is shown in Figure 1 . Figure 1. The Mollier diagram, shown in Figure A-1 , is a chart on which enthalpy (h) versus entropy (s) is plotted. It is sometimes known as the h-s diagram and has an entirely different shape from the T-s diagrams. The chart contains a series of constant temperature lines,a series of constant pressure lines, a series of constant moisture or quality.
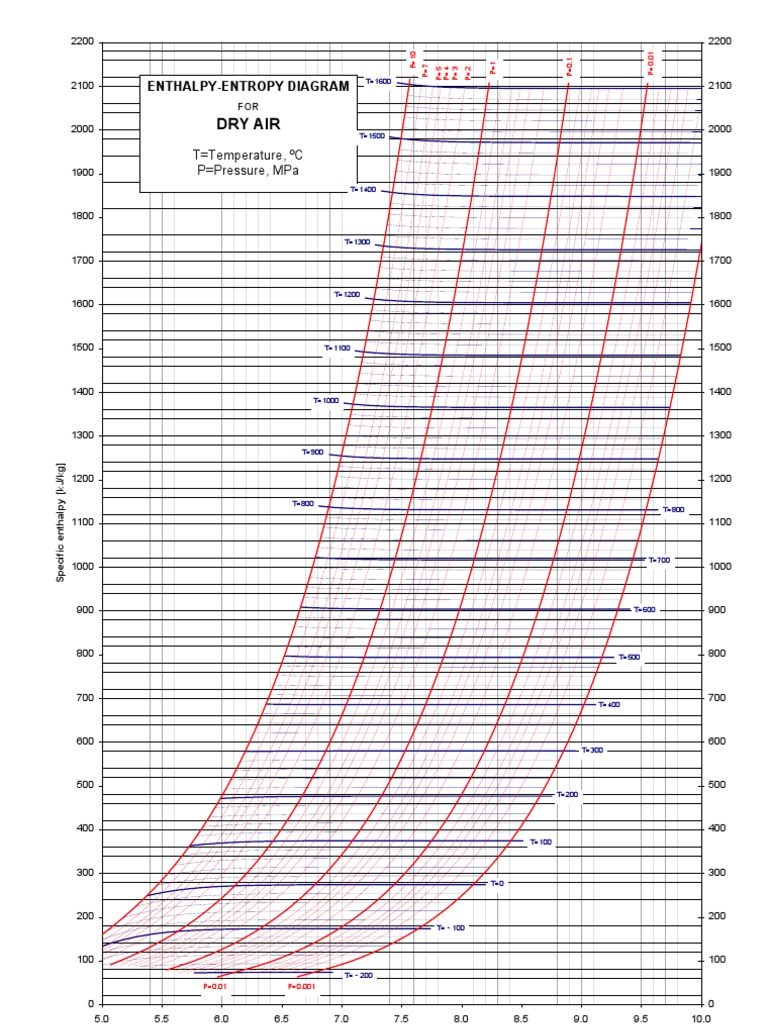
EnthalpyEntropy Diagram (Air) Enthalpy Statistical Mechanics
For full table with Entropy - rotate the screen! Water - Enthalpy and Entropy vs. Temperature - SI Units [kJ/kmol] [Btu (IT)/lb] [kJ/ (kmol K)] [kJ/ (kg K)] [kWh/ (kg K)] [Btu (IT)/lb [kcal/ (kg K)] 1 Btu (IT)/lb = 0.002326 GJ/t = 2.326 kJ/kg = 0.5559 kcal/kg = 0.000646 kWh/kg Enthalpy-entropy diagrams are presented for natural gasses of 0.6, 0.7, 0.8,0.9, and 1.0 gravity over the pressure range of 5 to 10,000 lb. per sq. in. andtemperature range of 32 degrees to 700 degrees Fahrenheit.




