Which element has the electron configuration of 1s2 2s2 2p4 ? Wayne Breslyn 727K subscribers Join Subscribe Subscribed 157 23K views 3 years ago To figure this out the element with the electron. What element has the electron configuration of 1s2 2s2 2p6 3s2 3p4? Sulfur (S). You can sum the number of electrons on all the subshells, and you will get the number 16. In its ground state, it corresponds to an atomic number that is unique to sulfur in the periodic table.

Chemistry291 Hand Note 【 】 1s2 2s2 2p4 What element has the electron
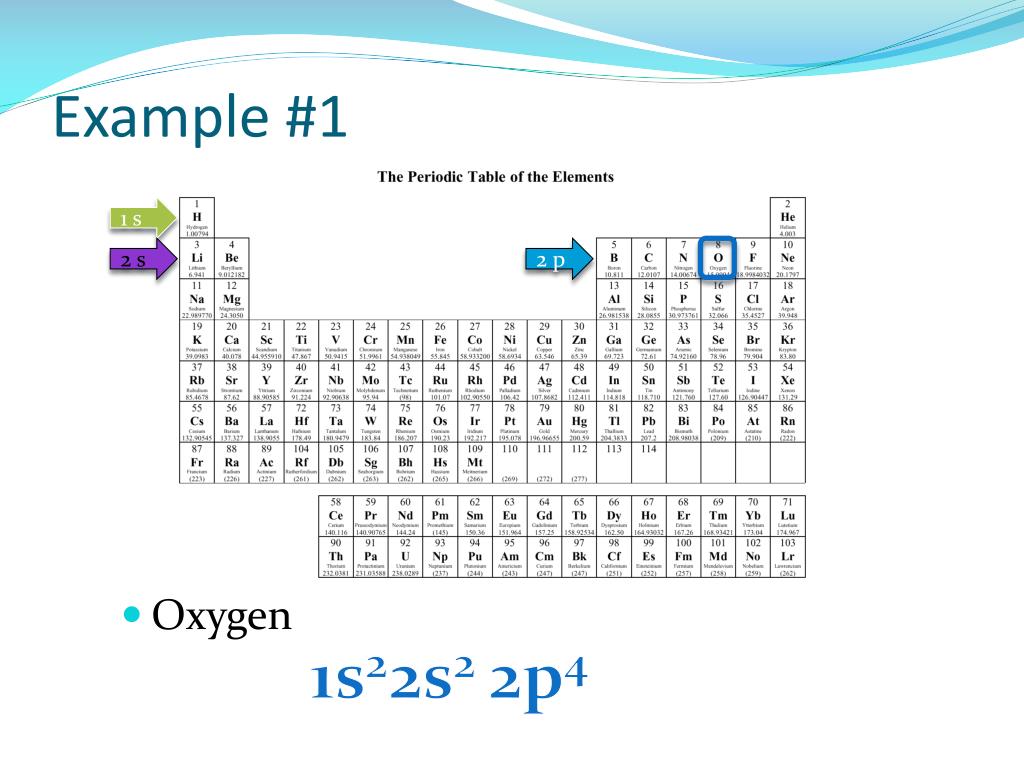
Key Questions How do electron configurations correspond to the periodic table? When looking at electron configuration, your fill order of electrons is: 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s etc. Group 1A (1), the alkali metals all end is s1. What period the element is in determines the 1st number. 1s2 2s2 2p4: Fluorine: 1s2 2s2 2p5: Neon: 1s2 2s2 2p6: Sodium: 1s2 2s2 2p6 3s1: Magnesium: 1s2 2s2 2p6 3s2: Aluminum: 1s2 2s2 2p6 3s2 3p1: Sulfur: 1s2 2s2 2p6 3s2 3p4: Chlorine: 1s2 2s2 2p6 3s2 3p5: Argon: 1s2 2s2 2p6 3s2 3p6: Potassium: 1s2 2s2 2p6 3s2 3p6 4s1: Calcium: 1s2 2s2 2p6 3s2 3p6 4s2: Mr. Vissers. Send e-mail; Oxygen's electron configuration is 1s2 2s2 2p4. We know it has 8 total electrons, and 6 in its outermost shell. Just as with nitrogen, the 1s and 2s orbitals will be filled, but we need to use Hund's Rule to fill the 2p orbital correctly. Question Electronic configuration of first 30 elements with table full explanation Solution H (Hydrogen) 1s1 He (Helium) 1s2 Li (Lithium) 1s2 2s1 Be (Beryllium) 1s2 2s2 B (Boron) 1s2 2s2 2p1 C (Carbon) 1s2 2s2 2p2 N (Nitrogen) 1s2 2s2 2p3 O (Oxygen) 1s2 2s2 2p4 F (Fluorine) 1s2 2s2 2p5 Ne (Neon) 1s2 2s2 2p6 Na (Sodium) 1s2 2s2 2p6 3s1

2S1 2/2 YouTube
#1 viper2308 19 0 Homework Statement Which of the following electron configurations correspond to an excited state? Identify the atoms and write the ground-state electron configuration where appropriate. 1s2 2s2 2p4 3s1 [Ar]4s2 3d5 4p1 Homework Equations none The Attempt at a Solution I have no idea what to do. What is the atomic number of the element with an electron configuration of 1s2 2s2 2p4? ? 3 ? 4 ? 8 ? 13; What element has the configuration 1s2 2s2 2p1?. How many inner-shell electrons does the following configuration indicate: 1s2 2s2 2p6 3s2 3p3? ? 3 ? 10 ? 12 ? 15; Which letter is used to indicate the angular momentum quantum number?. For example, the electronic configuration of oxygen (atomic number 8) is 1s2 2s2 2p4. This indicates that oxygen has two electrons in the 1s subshell, two electrons in the 2s subshell, and four electrons in the 2p subshell.. indicating that it has 12 electrons. The electronic configuration of magnesium is: 1s2 2s2 2p6 3s2. This configuration. The ground-state electron configurations of the elements are listed in Table 9.9.9 B. 1. The "exceptions" to the simple mnemonic noted in general chemistry texts are partly a consequence of the inadequacy of a "one-orbital order-fits-all" model. For example, copper has an electron configuration of [Ar]4s 1 d 10.
PPT Atomic Structure and Bonding PowerPoint Presentation, free
Science Chemistry Chemistry questions and answers How many valence electrons are there in an element with the following electron configuration? 1s2 2s2 2p4 _______ has ____________ valence electrons This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Sulfur is a 'group 16' element (electronic configuration: [Ne] 3s2 3p4) and Oxygen is also a 'group 16' element (electronic configuration: 1s2 2s2 2p4) in Periodic table. To draw the electron dot structure we count all the outer most shell electrons that participate in the molecule formation. Both Sulfur and Oxygen has two less.
Sulfur is a nonmetal element with an atomic number of 16. This means that it has 16 protons in its nucleus. The sulfur electron configuration lists the different ways that sulfur can arrange its electrons. The most common sulfur electron configuration is 1s2 2s2 2p6 3s2 3p4. This means that the sulfur atom has two electrons in the first energy. This Electron Configuration Full table gives the Electron Configuration Full of all the elements of periodic table . Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Electron Configuration Full ' headers to sort. Periodic Table of Elements with Electron Configuration Full Trends

[Solved] Na O I I S Si 1s2 2s2 2p4 1s2 2s2 2p6 3s2 3p4 1s2 2s2 2p6 3s2
1s2 2s2 2p4 ||Which element has the electron configuration of 1s2 2s2 2p4 ?#1s22s22p4 The excited state electron configuration of an atom indicates the promotion of a valence electron to a higher energy state. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. Example The ground state electron configuration of sodium is "1s"^2"2s"^2"2p"^6"3s"^1. In its excited state, the valence electron in the "3s.



