8.1.1 E2 Mechanism . E2 mechanism is the bimolecular elimination mechanism, that the reaction rate depends on the concentration of both substrate and base.We will take the elimination reaction of 2-bromo-2-methylpropane as an example for discussion. Figure 8.1a Bimolecular Elimination Reaction It was mentioned earlier that HX is the side product of dehydrohalogenation, why there is no HX (HBr. It could be understood as that with the presence of excess base (OH-) in the reaction mixture, HBr reacts with OH- to give H2O and Br-. The following discussion of the mechanism will help you to understand this better.

PPT Elimination Reactions PowerPoint Presentation, free download ID
In the other (bottom) pathway, methoxide ion acts as a base (rather than as a nucleophile) in an elimination reaction. As we will soon see, the mechanim of this reaction is single-step, and is referred to as the E2 mechanism. In the methanol solvent used here, methanethiolate has greater nucleophilicity than methoxide by a factor of 100. 5. By definition, an E1 reaction is a Unimolecular Elimination reaction. This means the only rate determining step is that of the dissociation of the leaving group to form a carbocation. Since E2 is bimolecular and the nucleophilic attack is part of the rate determining step, a weak base/nucleophile disfavors it and ultimately allows E1 to. elimination reaction: R2CH-CBrR2 + NaOH ==> R2C=CR2 + H2O + NaBr as well as/versus the substitution reaction: R2CH-CBrR2 + NaOH ==> R2CH-C (OH)R2 + NaBr In both cases R = H, alkyl or aryl and 'HBr' is eliminated or 'Br' substituted. For instance, the base-induced elimination of HBr from 1-bromo-2-phenylethane proceeds 7.11 times faster than the corresponding elimination of DBr from 1-bromo-2, 2-dideuterio-2-phenylethane. This result tells us that the C-H (or C-D) bond is broken in the rate-limiting step, consistent with our picture of the E2 reaction as a one-step process.

PPT Elimination Reactions PowerPoint Presentation, free download ID
Elimination Reactions 1. The E2 Reaction. We have not yet considered the factors that influence elimination reactions, such as example 3 in the group presented at the beginning of this section. (3) (CH 3) 3 C-Br + CN (-) ——> (CH 3) 2 C=CH 2 + Br (-) + HCN We know that t-butyl bromide is not expected to react by an S N 2 mechanism. . Furthermore, the ethanol solvent is not sufficiently. 1. The C-Br bond is relatively weak (<300kJ/mol) compared to other C-X bonds. The C-I bond is even weaker. The kinetic energy supplied by room temperature is enough to get the Br to spontaneously dissociate. 2. Br is a large atom, with lots of protons and electrons. Leaving groups need to accept a lone pair of electrons when they leave. In recent studies, Ohgiya, Nishiyama, et al. and we have explored the DBU-promoted regioselective HBr-elimination of vicinal dibromides having an adjacent O-functional group. 3(g), 6 A typical example is illustrated in Scheme 1, which shows that both the elimination reactivity and regioselectivity are affected by the presence or absence of the neighboring O-functional group in the substrate. Suggest a mechanism to explain the formation of (chloromethyl)benzene from methylbenzene. Strategy Draw out a balanced chemical equation for this reaction, and work out what the driving force for this process is. This reaction is more than likely a radical process as it requires UV radiation.

Intrinsic reaction coordinate energies for HBr elimination via 3centre
Chemical activation experiments and computational methods have been used to study the unimolecular reactions of C2H5CH2Br and C2D5CHFBr with 90 and 93 kcal mol-1 of vibrational energy, respectively. The four-centered elimination reactions of HBr and DBr are the dominant reactions; however, 2,1-DF, 1,1-HBr, and 1,1-HF reactions are also observed from C2D5CHFBr. The main focus was to search. Chapter First Online: 04 January 2022 953 Accesses Abstract Elimination reactions are the reverse of addition reactions. These involve elimination of two or four groups attached to adjacent carbon atoms in a substrate forming a multiple bond. Download chapter PDF 1 Introduction Elimination reactions are the reverse of addition reactions.
Introduction to Elimination Reactions. As part 4 of the most important reactions you learn in org 1, (acid-base, substitution, and addition) here's an introduction to the elimination reaction. This series requires that you understand how to read line diagrams ( click for video introduction) as well as to understand what wedge-dash notation. One of the first proposals for the mechanism of the E2 reaction. Prof. Ingold mentions in this paper, " It follows from the basic hypothesis that the ease of removal of the b-proton (reaction A) depends (a) on its vulnerability, (b) on the proton-avidity of the attacking anion". Influence of poles and polar linkings on the course pursued by.
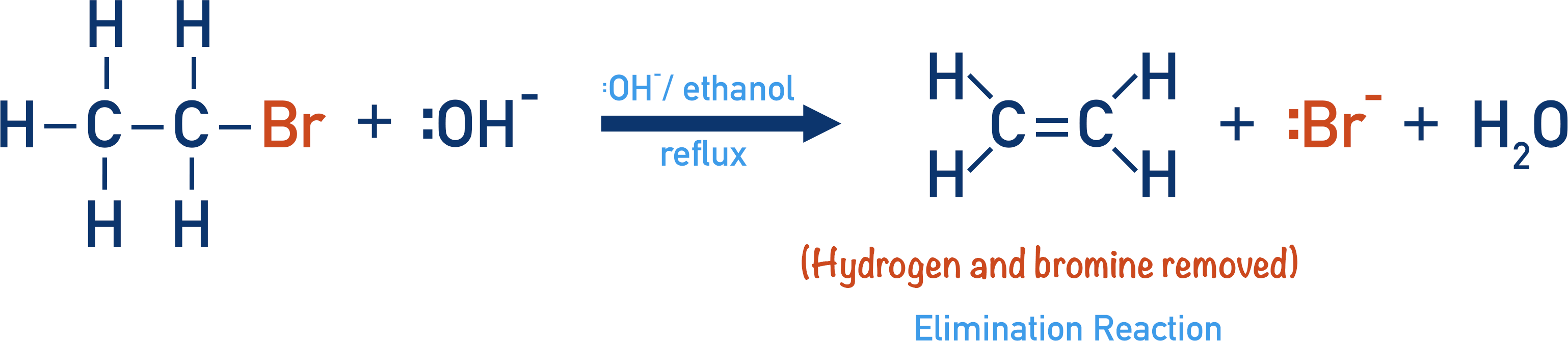
Halogenoalkanes Elimination Reactions (ALevel) ChemistryStudent
Master Organic Chemistry Reaction Guide Addition of HBr to Alkenes Description: Treatment of alkenes with hydrobromic acid will result in the formation of alkyl bromides. Notes: This is an addition reaction. Note that the bromine always ends up at the more substituted carbon of the alkene (Markovnikoff-selectivity) Examples: E1 elimination - An overview. The reverse of electrophilic addition is called E1 elimination. We will begin by looking at some non-biochemical E1 reactions, as the E1 mechanisms is actually somewhat unusual in biochemical pathways. E1 elimination: An E1 elimination begins with the departure of a leaving group (designated 'X' in the general.




