A step-by-step explanation of how to draw the Acetone Lewis Structure. Acetone is a member of the Ketone family of organic compounds. It is the simplest. 0:00 / 2:07 A step-by-step explanation of how to draw the (CH3)2CO Lewis Dot Structure (Acetone).For the (CH3)2CO structure use the periodic table to find the total numb.

Acetone Formula C3H6O Structure, Formula, Uses Embibe
Acetone ( 2-propanone or dimethyl ketone) is an organic compound with the formula (CH3)2CO. [22] It is the simplest and smallest ketone ( >C=O ). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. Written by Priyanka in Lewis Structure The chemical formula C3H6O represents acetone. This compound is also referred to as propanone or, in some texts as Propan-2-one. Acetone is considered to be the simplest form of Ketone. This compound is colorless, flammable, and is pungent smelling. It boils at temperatures of approximately 56° C. Geometry of Molecules 3.23K subscribers Subscribe Subscribed 4.3K views 1 year ago Lewis Structure CH3COCH3 is a chemical formula for acetone. And to help you understand the Lewis. For the Lewis structure for Acetone, calculate the total number of valence electrons for the Acetone molecule. After determining how many valence electrons there are in Acetone, place them around the central atom to complete the octets. There are a total of 24 valence electrons in the Lewis structure for Acetone. It is helpful if you:

Acetone Formula Properties, Preparation & More Embibe
Steps Here's how you can easily draw the acetone Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure ChemSpider Search and share chemistry For medical information relating to Covid-19, please consult the World Health Organisation or local healthcare provision. Simple Structure Advanced History Comment on this record 3D Acetone Molecular Formula CHO Average mass 58.079 Da Monoisotopic mass 58.041866 Da ChemSpider ID 175 More details: Acetone: The simplest ketone . Also polar ( ε = 21) aprotic solvent of molecular formula C 3 H 6 O. Also called 2-propanone or di methyl ketone. Molecular Structure of Acetone The C3H6O Lewis structure is a molecular formula that represents a compound with three carbon atoms, six hydrogen atoms, and one oxygen atom. The structure of the molecule is such that the carbon atoms are arranged in a chain with the oxygen atom bonded to one of the end carbon atoms. Each carbon atom in the chain is also bonded to two hydrogen.
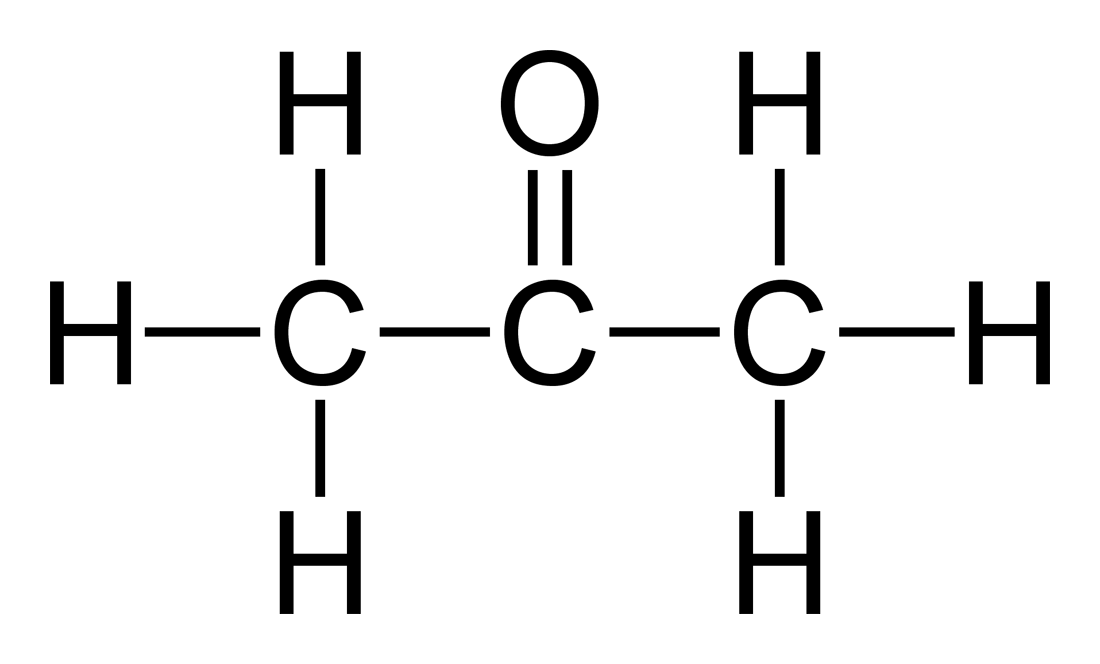
Acetone Formula (C3H6O) Structural and Organic Formula of Acetone (Propanone)
Structural Formula. C 3 H 6 O. acetone . 2-propanone . Molecular Model Application loaded.. Lewis structure of Acetone (also known as Propanone or C3H6O) contains three Carbon atoms (C) in a row which have an Oxygen atom (O) attached to a central Carbon atom (C) forming a double bond. The outer Carbon atoms (C) are surrounded by the Hydrogen atoms (H). The Oxygen atom has 2 lone pairs.
The Acetone Lewis structure is an example of an organic functional group called a Ketone. The Ketone functional group is made up on three Carbon atoms with an Oxygen atom double bonded to the center Carbon atom. The Lewis structure for Acetone is the simpliest Ketone possible. Steps of drawing Acetone (C3H6O) lewis structure Step 1: Find the total valence electrons in C3H6O molecule. In order to find the total valence electrons in a C3H6O molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Acetone Formula C3H6O Structure, Formula, Uses Embibe
The Lewis structure of acetone molecule is a way to present the outermost valence electrons that are involved in chemical bonding. C3H6O is a trigonal planer molecule. It consists of three carbon, six hydrogen, and one oxygen atoms. © 2023 Google LLC Acetone is (CH3)2CO. aka C3H6O. here I show you how to draw its Lewis Structure.It's pretty straightforward.I'm not exactly sure why it's a challeng.




