Atom Modeli 3D (Atom Model) YouTube
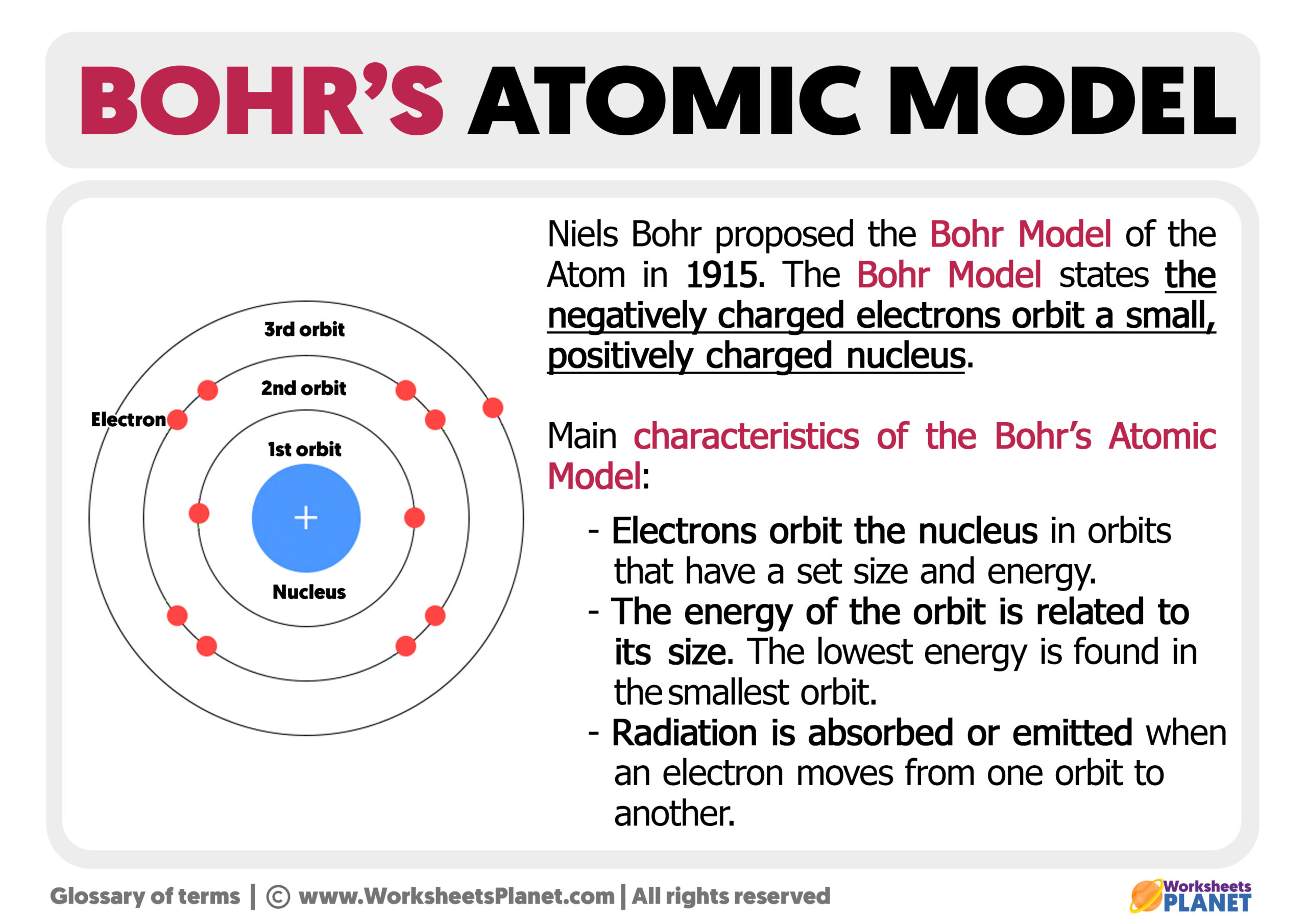
A model of a helium atom shows a black circle which fades to white moving from the center to the outside. At the center of the circle is a tiny nucleus, consisting of two red circles and two purple circles.. A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus is labelled n=1. How does Niels Bohr's atomic model work? An overview of Niels Bohr's refinement of the Rutherford model. See all videos for this article Bohr model of the atom In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n. Figure \(\PageIndex{5}\): In Bohr's Model of the atom, electrons absorb energy to move to a higher level and release energy to move to lower levels. (CC BY-SA 3.0; Kurzon). Bohr's Model and Atomic Spectra. The evidence used to support Bohr's model came from the atomic spectra. He suggested that an atomic spectrum is made by the electrons in an. Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.
Figure 6.2.1 6.2. 1: Quantum numbers and energy levels in a hydrogen atom. The more negative the calculated value, the lower the energy. We can relate the energy of electrons in atoms to what we learned previously about energy. The law of conservation of energy says that we can neither create nor destroy energy. He was struggling to make sense of all of this. As was common with Bohr when confronted with a puzzle, this struggle was nearly all-consuming. Then in 1913 Bohr, by accident, stumbled across Balmer's numerology for the hydrogen spectrum, and in a flash came up with a workable model of the atom. The model asserts that: The planetary model is. Video \(\PageIndex{1}\): An introduction to the Bohr Model of the Atom. In 1913, Niels Bohr attempted to resolve the atomic paradox by ignoring classical electromagnetism's prediction that the orbiting electron in hydrogen would continuously emit light. Instead, he incorporated into the classical mechanics description of the atom Planck's ideas of quantization and Einstein's finding that. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
3 boyutlu hidrojen atom modeli
In 1913, Danish physicist Niels Bohr applied Max Planck's quantum theory to the nuclear atom of Ernest Rutherford, thus formulating the well-known planetary model of the atom, wherein electrons orbit a central nucleus in well-defined levels of energy ().Note that Bohr stated that electrons in the atom follow elliptical orbits (not circles as is often pictured). 🎈 12. Sınıf Konuları Serisinin Kitabını Sipariş Etmek İçin : https://bit.ly/3xb9oqW🔥 Bu Serinin Oynatma Listesi İçin : https://bit.ly/3B7HFIS🚀 TYT. Resources. Lecture Slides (PDF - 9.3MB) Periodic Table and Table of Constants. Lecture Summary. Prof. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom.He details Bohr's postulates for the hydrogen atom and discusses how the Planck-Einstein relationship applies to electron transitions. ATOM MODELİ YAPILIŞI.Basit Atom Modeli yapımı.Atom modeli nasıl yapılır?Rutherford,Bohr.