Propanone is normally written CH 3 COCH 3. Notice the need for numbering in the longer ketones. In pentanone, the carbonyl group could be in the middle of the chain or next to the end - giving either pentan-3-one or pentan-2-one.. Both aldehydes and ketones are polar molecules because of the presence of the carbon-oxygen double bond. As well. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than alcohols. Then again, acetone (and other carbonyl containing solvents) are, indeed, poor solvents when using strong bases due to their relatively high acidity.
GCSE Chemistry C3 Organic Chemistry Revision Cards in GCSE Chemistry
To determine if C3H6O (Acetone) is a polar or non-polar molecule we need to look at the Lewis structure, molecular geometry, and the electronegativity of the atoms in C3H6O..more.more. Acetone ( 2-propanone or dimethyl ketone) is an organic compound with the formula (CH3)2CO. [22] It is the simplest and smallest ketone ( >C=O ). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. This compound is also referred to as propanone or, in some texts as Propan-2-one. Acetone is considered to be the simplest form of Ketone. This compound is colorless, flammable, and is pungent smelling. It boils at temperatures of approximately 56° C. Polarity of Solvents. Water Acetic Acid Ethyleneglycol Methanol Ethanol Isopropanol Pyridine Acetonitrile Nitromethane Diehylamine Aniline Dimethylsulfoxide Ethylacetate Dioxane Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethylether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum ether Hexane Pentane.

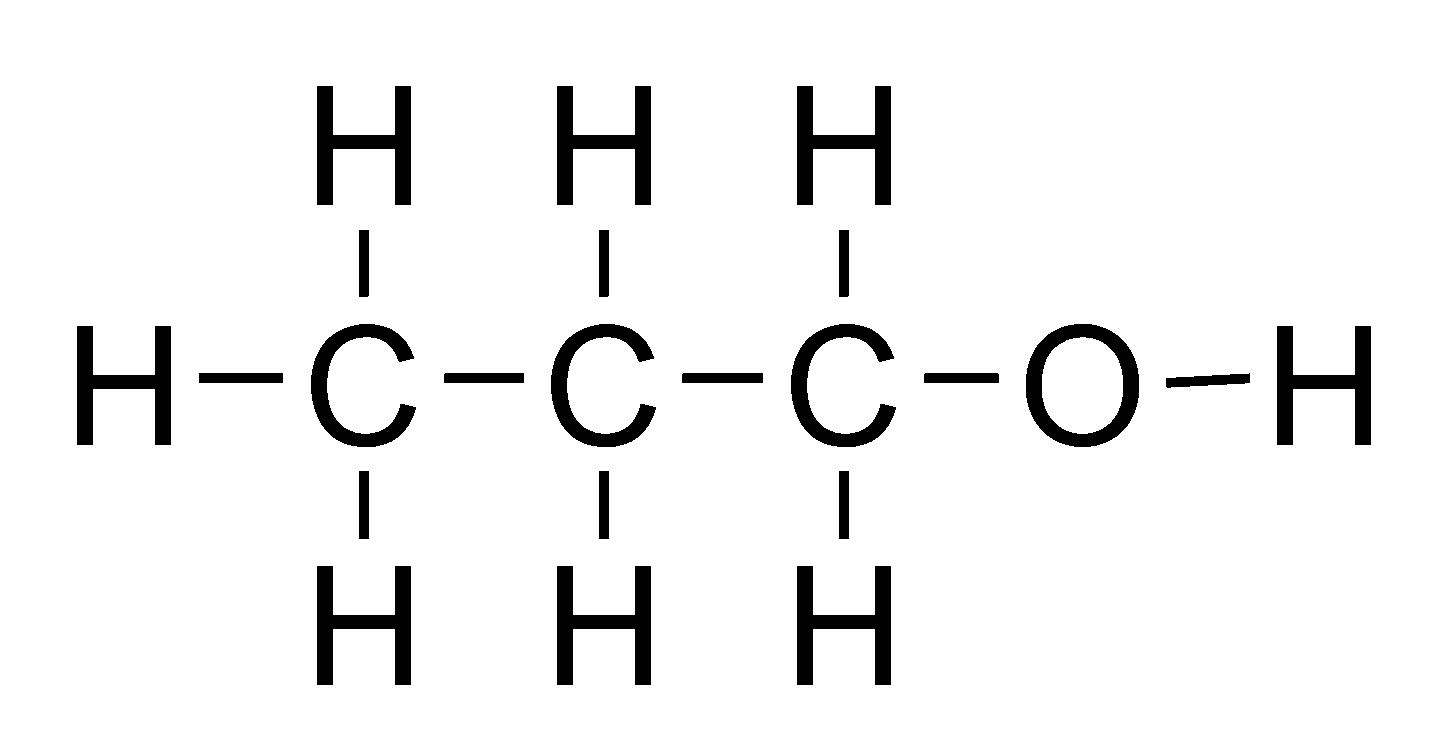
Lewis Structure Of 1 Propanol
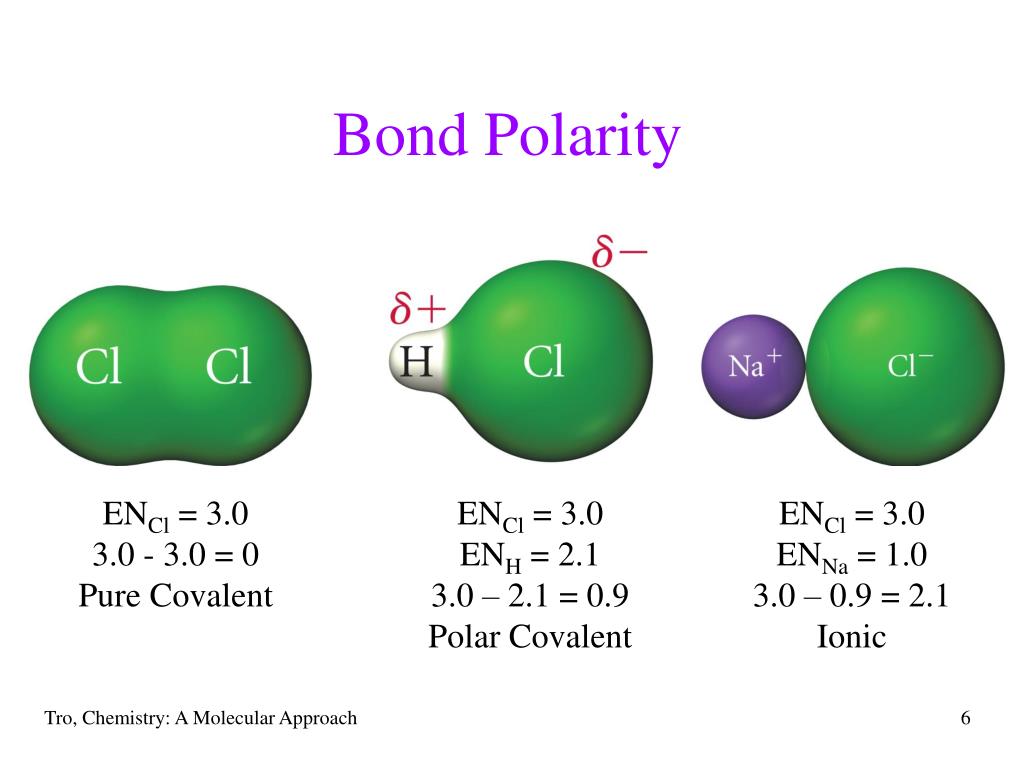
5. "Borderline" Polar Aprotic Solvents Have Small Dipole Moments And Low (<10) Dielectric Constants. These solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Since they have intermediate polarity they are good "general purpose" solvents for a wide range of reactions. The carbonyl group is rather polar, however, since the difference between the electronegativities of carbon (2.5) and oxygen (3.5) is rather large, and there are usually no other dipoles in an aldehyde or ketone molecule to cancel the effect of C==O.. Like other ketones, acetone is mainly useful as a solvent, and you may have used it for. The Rf value varies depending on the solvent used, but the general order of the pigments (from the highest to the lowest Rf value) usually remains the same, because the nonpolar compounds move further than the polar compounds. Rf values for various pigments (using hexane, acetone and trichloromethane (3:1:1) for the solvent) are shown in table 1. To Your Health: Acetone in Blood, Urine, and Breath. Acetone is formed in the human body as a by-product of lipid metabolism. Normally, acetone does not accumulate to an appreciable extent because it is oxidized to carbon dioxide and water. The normal concentration of acetone in the human body is less than 1 mg/100 mL of blood.

DP Chemistry Iodination of propanone
Propanone is SLIGHTLY POLAR and so can dissolve other polar substances-Propanone is LESS POLAR than water-this allows a RESOLUTION resolution between the pigment and the TLC plate as the pigments will dissolve in the propanone. Common solvents arranged from the least polar to the most polar. Solvent Relative Polarity; hexane 0.009 p-xylene 0.074 toluene 0.099 benzene 0.111 ether 0.117 methyl t-butyl ether (MTBE). acetone 0.355 dimethylformamide (DMF) 0.386 t-butyl alcohol 0.389 sulfolane 0.41 dimethylsulfoxide (DMSO) 0.444 acetonitrile 0.46 nitromethane 0.481.
∙ 11y ago Study now See answer (1) Best Answer Copy Propane itself is non polar, but the presence of the ketone group (C=O) in propanone makes it a polar molecule (oxygen has partial -ve. 217-218 °C Alfa Aesar: 218 °C Food and Agriculture Organization of the United Nations 1-Phenyl-1-propanone: 218 °C OU Chemical Safety Data (No longer updated) More details: 217-218 °C Alfa Aesar A15140: 218 °C Oakwood: 8 °C / 84 mmHg (68.4982 °C / 760 mmHg) FooDB FDB010567 218 °C Sigma-Aldrich SIAL-61074: 218 °C Oakwood 094675
Ppt Polar Bonds And Molecules Powerpoint Presentation, Free Download 587
- Techiescientist Is Acetone Polar or Nonpolar? Acetone is an organic compound with its chemical formula (CH3)2CO. It is classified as the simplest ketone. It exists as a colorless volatile liquid. It has a characteristic odor and flammable in nature. Many students may have a question regarding whether acetone is polar or not. Propanone is very soluble in water because а. it is non-polar b. the dipole-dipole interactions are weak between propane and water С. it can form hydrogen bond with water molecule d. water is a polar solvent Chemistry: The Molecular Science 5th Edition ISBN: 9781285199047 Author: John W. Moore, Conrad L. Stanitski



