If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. If there are more electrons than protons, the ion has a negative charge and is called an anion. Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons for heavier. An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885-1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.. 3D diagram of circular 1s.
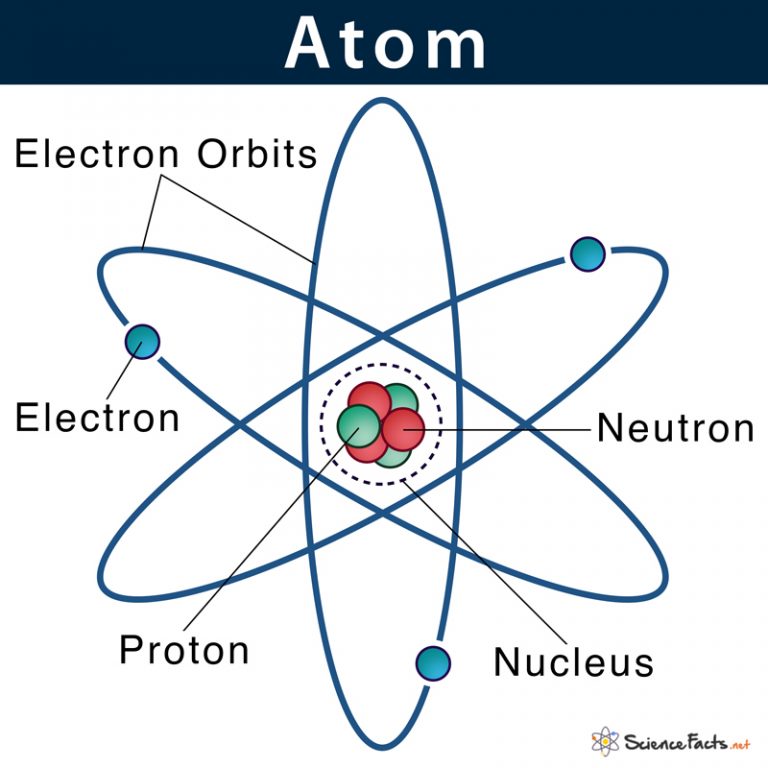
Atom Definition, Structure & Parts with Labeled Diagram
Most of the atom is empty space. The rest consists of three basic types of subatomic particles: protons, neutrons, and electrons.The protons and neutrons form the atom's central nucleus. (The ordinary hydrogen atom is an exception; it contains one proton but no neutrons.) As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry. Basic Diagram of an Atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. When one says an atom is electrically neutral, it means that the number. Physical Chemistry (Essentials) - Class 11 8 units · 52 skills. Unit 1 Welcome to physical chemistry. Unit 2 Structure of atom. Unit 3 Some basic Concepts of Chemistry. Unit 4 Redox reactions. Unit 5 Gaseous state. Unit 6 Thermodynamics. Unit 7 Chemical Equilibrium. Unit 8 Ionic equilibrium. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise 2.2.1 2.2. 1. An ion of platinum has a mass number of 195 and contains 74 electrons.
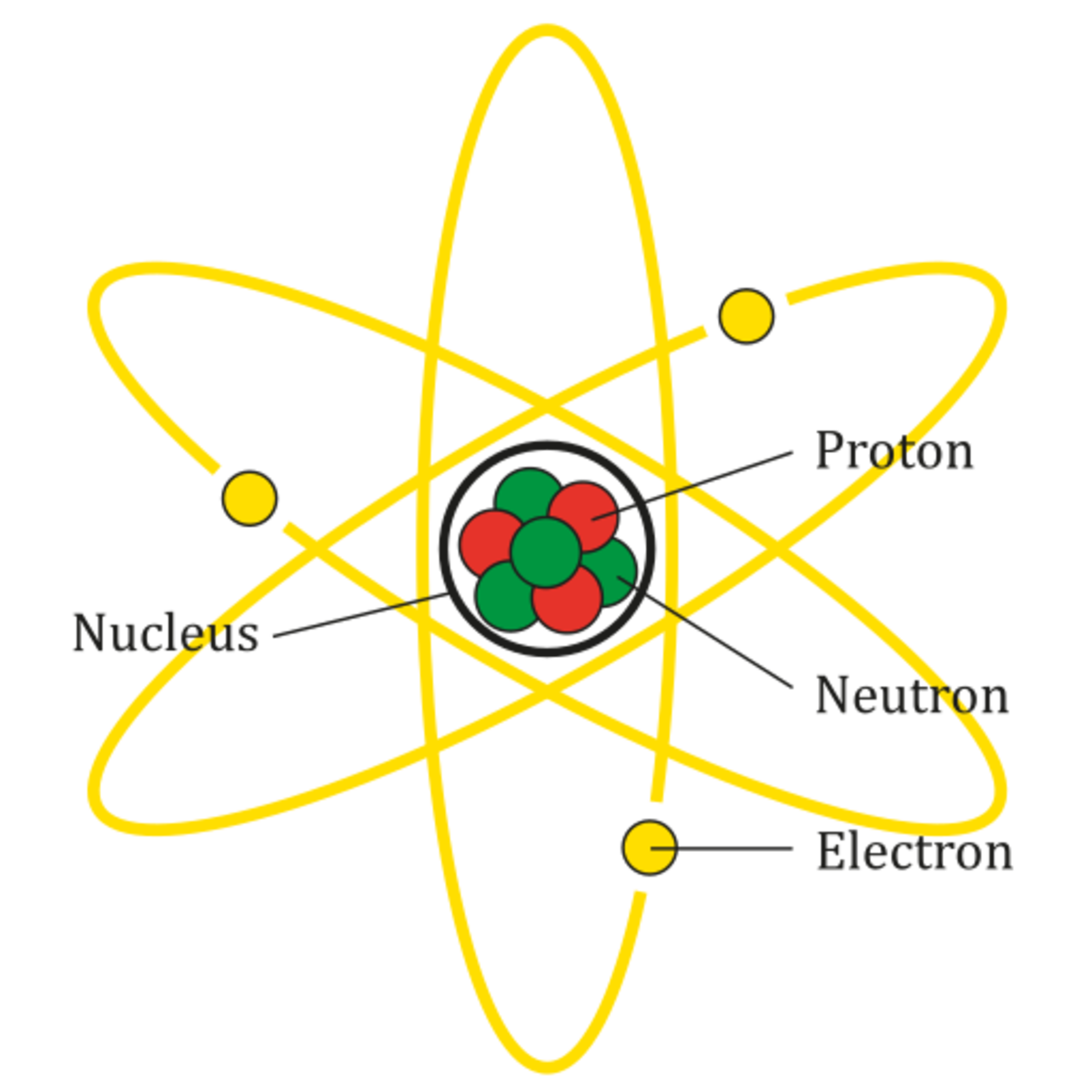
Atoms, Molecules, and Compounds What's the Difference? Owlcation
Atom. Atoms are tiny particles that form the basic building blocks of all matter in the universe, whether solid, liquid, or gas. All living organisms and nonliving objects found on Earth are made of trillions and trillions of atoms. The smaller particles that make up an atom are known as subatomic particles. The term 'atom' was derived from. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is. An atom has a central nucleus close nucleus The central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. The plural of nucleus is nuclei..This is surrounded. 1.14: The Nuclear Atom. Describe the history of the atom. Draw a diagram of a model of the atom and label the nucleus and the electron cloud. The history of the atom begins before 1800, where an atom was thought to be the smallest piece of matter. The first scientist to provide a theory about the atom was John Dalton.

Atomic Structure Broad Learnings
Diagram of alpha and beta decay in two Uranium isotopes. Credit: energy-without-carbon.org. Nuclear fission, where an atom of Uranium 92 is split by a free neutron to produce barium and krypton. 5.8: Orbitals. Page ID. Ed Vitz, John W. Moore, Justin Shorb, Xavier Prat-Resina, Tim Wendorff, & Adam Hahn. Chemical Education Digital Library (ChemEd DL) A characteristic of the diagram Figure 1 in Electron Waves in the Hydrogen Atom is that it has been assigned an identifying label, namely, 1 s.
1. Draw five protons in the nucleus of the atom. Label them with their charge. 2. Draw six neutrons in the nucleus of the atom. 3. Draw two electrons in the first energy level and label them with their charge. 4. Draw three electrons in the second energy level and label them with their charge. 5. What element is represented by the diagram? Atom Diagram [/caption]The image on the left is a basic atom diagram. This one shows the protons, neutrons, and electrons of a carbon atom. Each is in a group of six. That makes the atom very stable.
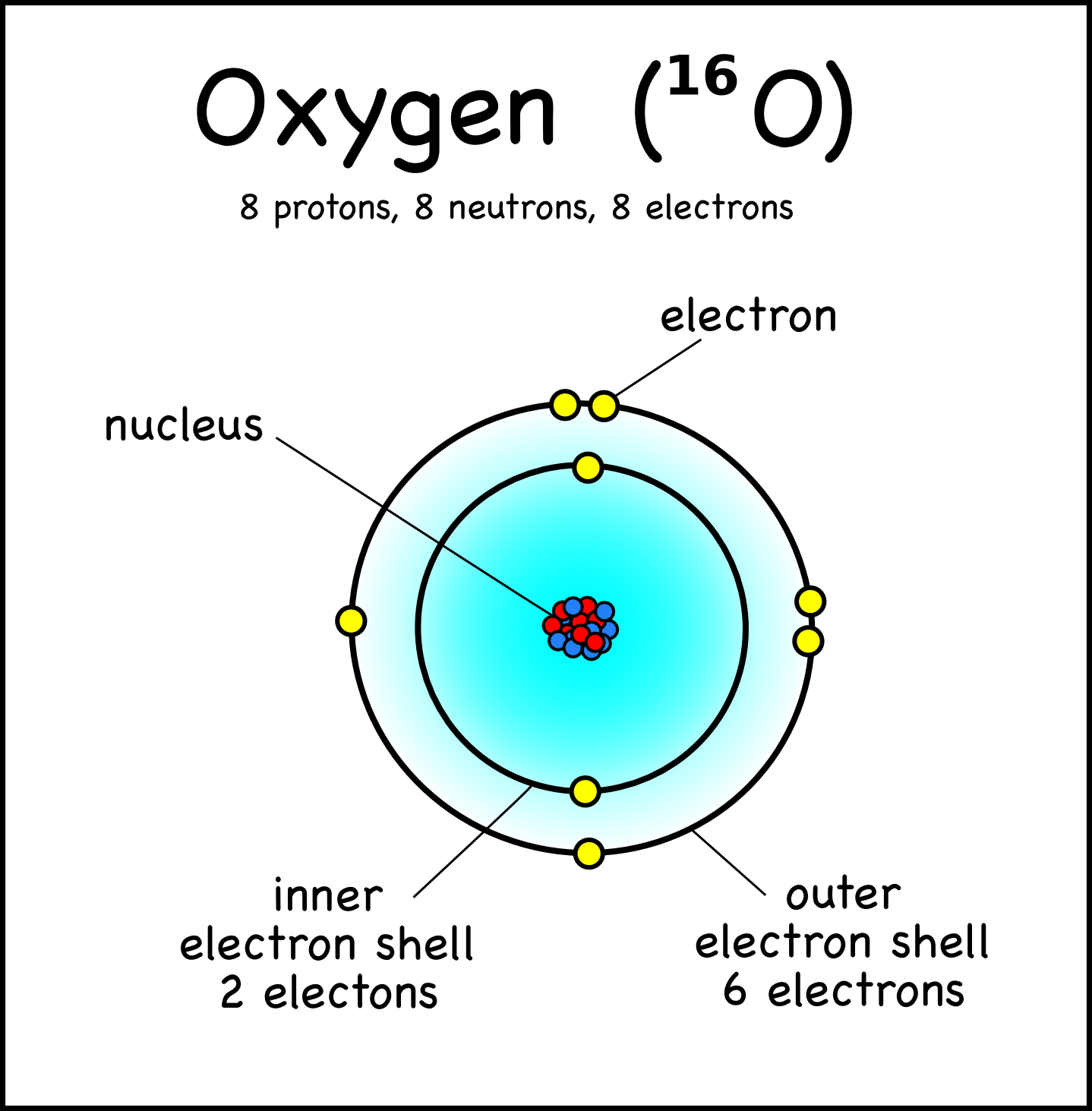
The Nucleus of the Atom and Radioactivity
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the. According to the diagram, this helium atom contains two protons, two neutrons, and two electrons. The numbers of protons and electrons make sense: the atomic number of helium is 2 , so any helium atom must have two protons in its nucleus (otherwise, it would be an atom of a different element!).And, because this is a neutral atom, it must contain two electrons to balance out the positive.




