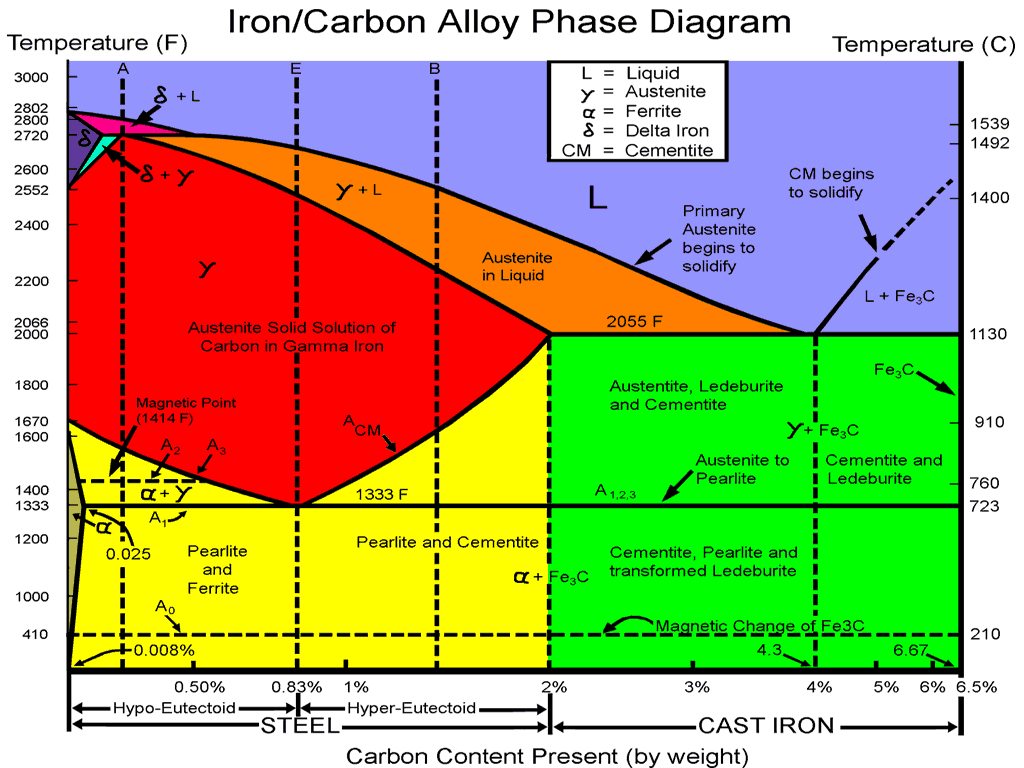
The iron-carbon phase diagram is widely used to understand the different phases of steel and cast iron. Both steel and cast iron are a mix of iron and carbon. Also, both alloys contain a small amount of trace elements. The diagram is also known as a "steel phase diagram" since iron alloys are steel. "Steel" is used in place of iron-carbon to show that the diagram is used to understand its microstructure. "Iron-iron carbide phase diagram" is used to represent the iron carbide part of the phase diagram.
Working the Steel
No phase diagram is more important to materials scientists than the Fe-C phase diagram because it allows us to explain many of the different types of steels.. The phase diagram illustrates the domains in which particular phases or combinations of phases are stable, and contains information about their equilibrium compositions. Equilibrium phase fractions can also be estimated from a knowledge of the carbon concentration of the steel and an application of the lever rule. Want to learn about steel phase diagrams? See our steel metallurgy courses The phases present in an alloy depend on the alloy composition and the thermal treatment to which the alloy has been exposed. Phase diagrams are graphical representations of the phases present in a particular alloy being held at a particular temperature. Thermodynamics tells us that: Under conditions of a constant temperature and pressure and composition, the direction of any spontaneous change is toward a lower free energy. The state of stable thermodynamic equilibrium is the one with equilibrium minimum free energy. A system at a metastable state is trapped in a local minimum of free

Steel Phase Diagrams 101 Diagrams
Summary of the phase transformations of steel - 07/02/2018 In this article, a summary is given about the phase transformations during solidification and cooling of steel. Iron-Carbon Phase Diagram | Creating | Steel | Cast Iron | hypo-eutectoid | hyper-eutectoid Watch on Introduction 1. Given the Fe-Fe3C phase diagram above, calculate the phases present at the eutectoid composition line at: a. T = 3000ºF b. T = 2200ºF c. T = 1333ºF d. T = 410ºF 2. Calculate the phases in the cast-iron portion of the diagram at the eutectic composition of 4.3% C in combination with 95.7% ferrite at: a. T = 3000ºF b. T = 1670ºF c. T = 1333ºF 3. For plain carbon steel with carbon concentrations below 2 %, you needn't worry, indeed. Graphite is never formed and the usual phase diagram covers everything nicely. For cast-iron , with carbon concentrations up to a few percent you need to worry. Graphite might form, depending on conditions. Advent of iron-carbon phase diagram was closely related to the concept of phase transition critical point of steel. In 1868, the Russian metallurgist D. K. Chernoff, by drawing on a great deal of practical work in forging, quenching, tempering, and fracture inspection of gun barrel, concluded that steel must be heated to above the temperature "a" so that it can be hardened after.

Steel Phase Diagrams 101 Diagrams
Abstract Steel is made by adding carbon to iron, producing a solid solution defined by its crystalline structure. This chapter discusses the effect of carbon composition and temperature on the types of structures, or phases, that form. Figure: Transformation lines in the iron-carbon phase diagram (steel part) Accordingly, three different types of steel can be distinguished, each of which undergo typical microstructural changes during cooling: eutectoid steels with a carbon content of exactly 0.8%! hypereutectoid steels with a carbon content greater than 0.8%!
8461 Phase transformations in steels can be compared to those of solid solutions (completely soluble) and crystal mixtures (completely insoluble). The figure below shows steel part of the iron-carbon phase diagram of the metastable system. Phase diagrams, also known as constitution diagrams or equilibrium diagrams, graphically represent the influences of alloy composition and temperature on phase changes and solidification. Figure 1

Steel Phase Diagrams 101 Diagrams
At the highest temperatures, the liquid phase field can be found, and below this are the two-phase fields (liquid + austenite, liquid + cementite, and liquid + delta-ferrite). In heat treating of steels, the liquid phase is always avoided. The steel portion of the Fe-C phase diagram covers the range between 0 and 2.08 wt. % C. Phase Diagrams. Module 1 • 58 minutes to complete. This course will explore higher-level details about phase diagrams, including the Fe-Fe3C phase diagram. We will use the Fe-Fe3C phase diagram to predict the possible phases and microstructures of a steel alloy based on its composition.




