Atomic Basics Answer Key Part A: Atomic Structure 1. Draw five protons in the nucleus of the atom. Label them with their charge. 2. Draw six neutrons in the nucleus of the atom. 3. Draw two electrons in the first energy level and label them with their charge. 4. Draw three electrons in the second energy level and label them with their charge. 5. 9.1095 ×10−28Kg 9.1095 × 10 − 28 K g. Unit electrical charge is 1.6022 ×10−19 1.6022 × 10 − 19 coulomb (C). The nucleus at the center of the atom contains one or more positively charged protons. All atoms of a given element have the same number of protons, which defines the element's atomic number, given the symbol Z Z.

Chemistry Atomic Structure Worksheet
Atomic structure P ast Paper Q uestions Science Exams Sorted 9. An atom of potassium has an atomic number of 19 and a mass number of 39. a. Complete the table to show the number of protons, neutrons and electrons in this. Give a reason for your answer.. Why Atoms Have Atomic Numbers on the Periodic Table. The atoms in each unique element have a specific number of protons. For example, oxygen has two atoms so its atomic number is 2. Iron has twenty-six protons in its nucleus so the atomic number is 26. Scientists can identify an element by its atomic number on the chart. Conclusion The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the atomic number. Worksheet 9: The Atomic Structures Topic 3 Set A: Objective: By defining these words, you will become more familiar with atomic structure related terms and their definitions. Define, neatly and clearly, the following atomic structure related terms. 1. Nucleus 2. Neutron 3. Proton 4. Electron 5. Nucleons 6. Atomic number 7. Mass number 8.

Atomic Structure Worksheet Answers Key Physical Science 54 FREE
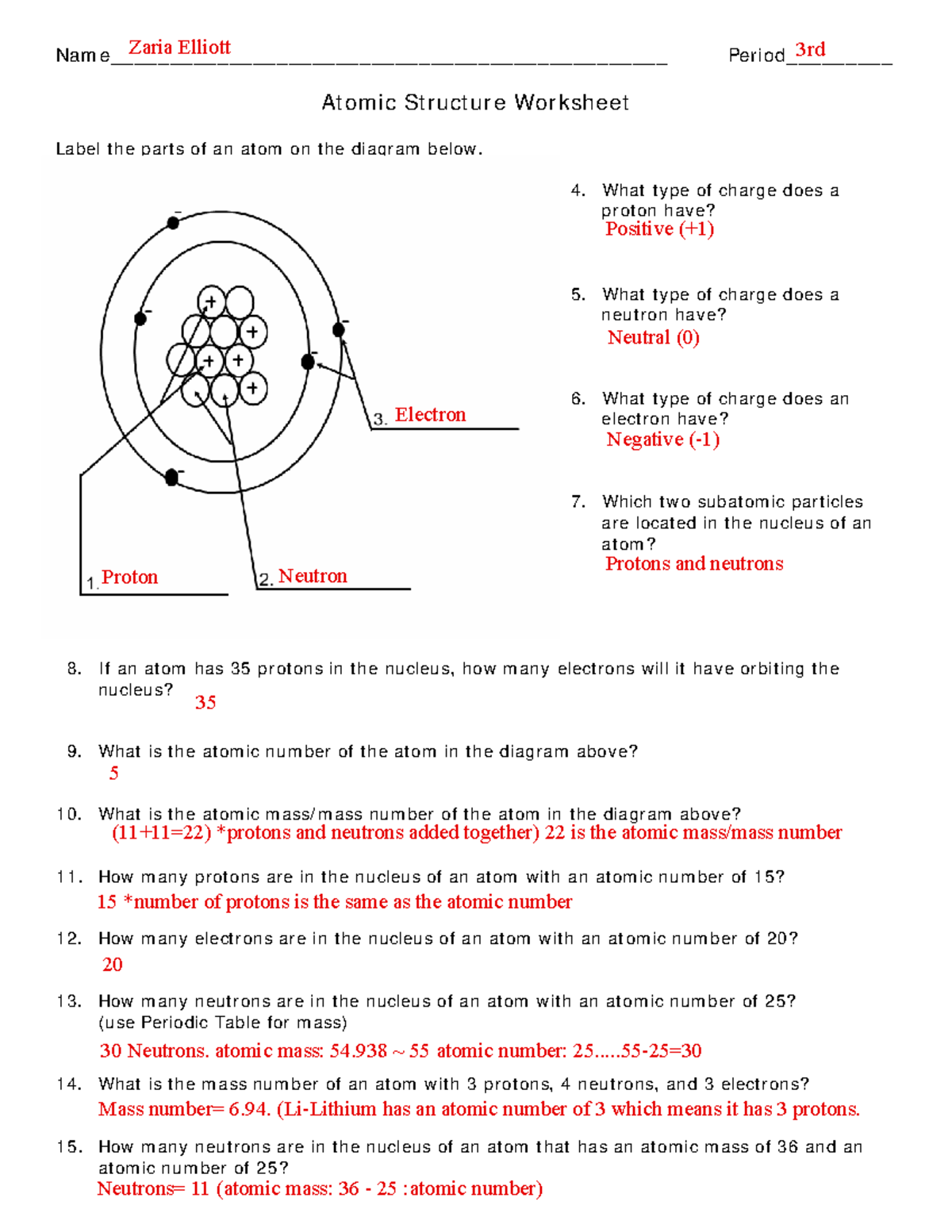
Z = atomic number [# of protons] N = # of neutrons A - Z = N A typical isotopic symbol takes this form: ex. The isotopic symbol for Fluorine would be Fill in the missing items in the table below. Fill in the missing items in the table below. Fill in the missing items in the table below. CHEMISTRY ATOMIC STRUCTURE PRACTICE I 17 Isotopic Symbol Na Atomic Structure Worksheet. Label the parts of an atom on the diagram below. 4. What type of charge does a proton have? 5. What type of charge does a. How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? Bohr Model Drawing (25 points) Draw a Bohr model of an . oxygen atom in the space. Atomic Structure Worksheet List the 3 subatomic particles, their charges, masses and where in the atom they are located in the chart below: Describe how to determine the number of each subatomic particle in an atom below Fill in the chart for the elements below. Round all atomic masses to the nearest whole number: Draw an atom below and label Worksheet: Atomic Structure Name_____ CHEMISTRY: A Study of Matter. 3.6 Use your notes from the Atomic Structure program to answer the following questions. 1. The atomic number tells the number of positively charged _____ in the nucleus of an atom. The atom is _____ because this is also the number. 3-06-Atomic Structure Wkst.doc

Ouille! 37+ Raisons pour Atomic Structure Worksheet Answers Key Pdf
Atomic Structure: Name: _____ Date: _____ B#: _____ Sketch An Atom Draw 5 protons in the nucleus and label with the charge. Draw 6 neutrons in the nucleus and label with the charge. Draw 2 electrons in the 1st energy level and label with their charge. Draw 3 electrons in the 2nd ndenergy level Atomic Structure Worksheet Name the three particles of the atom and their respective charges. Particle The nucleus has a Electrons are located in the Charge type charge and consist of Charge and charge. of an atom and carry The number of protons 'n one atom of an element determines the atom's and the number of electrons determines element.
What is the atomic mass of a sample of chlorine that has 19 neutrons? 8. What atom has 18 electrons? Example: Calculating the number of neutrons in an atom of lithium. +Atomic Structure Worksheet pages 10-11 This assignment is to be completed below in the space provided. 9. 10. 11. This Atomic Structure Worksheet with answer key PDF has loads of great activities to engage students with the atomic structure. Students can work through the card activities included, that will ask them to create the atomic structure, label and create a diagram. The PowerPoint is a fantastic resource that talks your students through the.
WK Number 2 Atomic Structure Chemistry 1 Worksheet Assignment with
(a) the ion with a 1+ charge, atomic number 55, and mass number 133 (b) the ion with 54 electrons, 53 protons, and 74 neutrons (c) the ion with atomic number 15, mass number 31, and a 3− charge 2. The Atomic Model Worksheet and Key 3. The Atomic Model of Matter Graphic Organizer and key 4. Atomic Model of Matter Worksheet and key 5. Atom Notes 6. Complete Model of Atom Graphic Organizer and Key 7. Vocabulary Review and Key 8. Periodic Table 9. Understanding the Atom - Finding Numbers of Protons, Neutrons, Electrons and Key 10.




