A step-by-step explanation of how to draw the C2H2Cl2 Lewis Dot Structure (1,2-Dichloroethene). For the C2H2Cl2 structure use the periodic table to find the total number of valence. C 2 H 2 Cl 2 Lewis structure is made up of two carbon atoms, two hydrogens, and two chlorine atoms. Both carbon atoms are kept at the central position and other atoms are at the surrounding position in lewis's structure. The lewis structure of C 2 H 2 Cl 2 contains one double bond (C=C) and four single bonds.
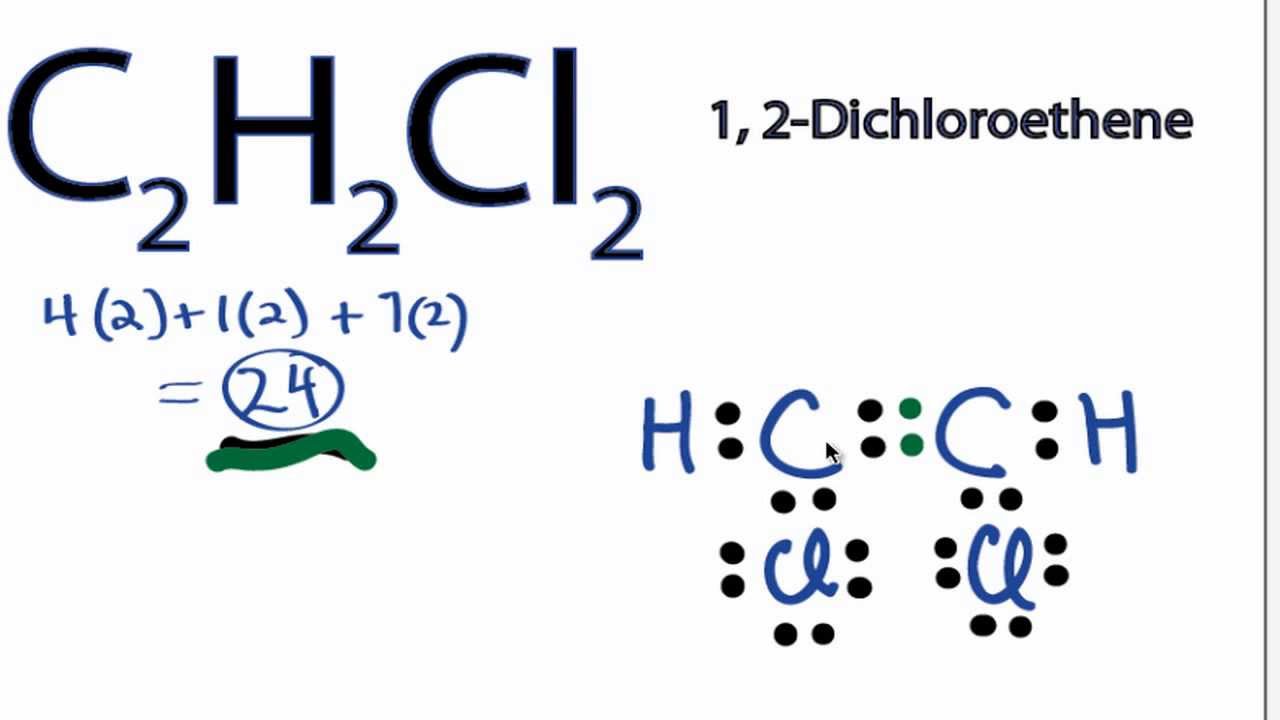
C2H2Cl2 Lewis Structure How to Draw the Lewis Structure for C2H2Cl2
Subscribed 1.2K views 1 year ago Lewis Structure CH2cl2 is a chemical formula for 1,2 dichloroethene. And to help you understand the Lewis Structure of this molecule, we are going to share. C2H2Cl2 (1, 2-dichloroethene) lewis structure has a double bond between the Carbon-Carbon atoms and a single bond between the Carbon-Hydrogen atoms and Carbon-Chlorine atoms. There are 3 lone pairs on Chlorine atoms (Cl). Lewis structure of C2H2Cl2 (1, 2-dichloroethene) contains a double bond between the two Carbon (C) atoms and a single bonds between Carbon-Hydrogen atoms and Carbon-Chlorine atoms. The Chlorine atom has 3 lone pairs. Let's draw and understand this lewis dot structure step by step. With C 2 H 2 Cl 2 there are only single bonds. Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Cl 2 Lewis structure. Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Note that Hydrogen only needs two valence electrons to have a full outer shell.

Lewis Dot Structure For C2h2cl2 Bauman Atten1980
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula C 2 H 2 Cl 2. They are both colorless liquids with a sweet odor. 1 Answer. These are the constitutional isomers (drawn in Lewis dot diagram notation) of C2H 2Cl2, It's basically aligning the chlorine and hydrogen atoms in different places around the molecule. The electron density is where the chlorine atoms are located. These are the constitutional isomers (drawn in Lewis dot diagram notation) of C_2H_2Cl_2. In the C 2 H 2 Cl 2 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with one hydrogen atom and one chlorine atom, and on each chlorine atom, there are three lone pairs. C2H2Cl2 Lewis Structure: How to Draw the Lewis Structure for C2H2Cl2. Watch on Contents Steps This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
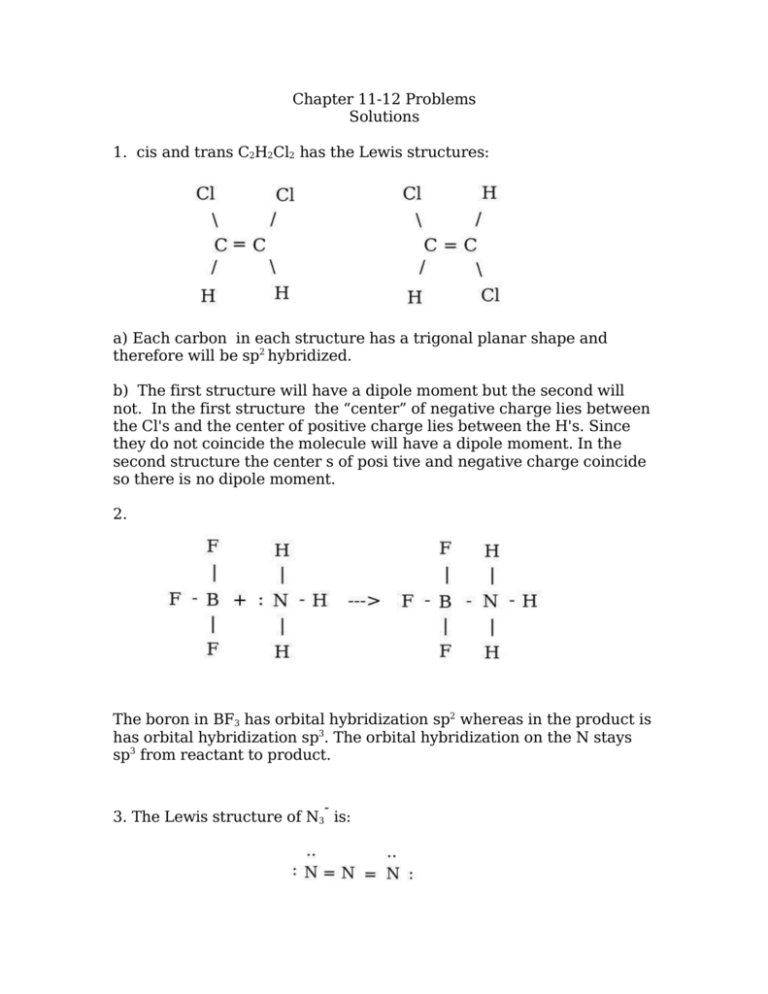
Chapter 1112 Problems Solutions 1. cis and trans C2H2Cl2 has the
1,1-Dichloroethene. Molecular Formula CHCl. Average mass 96.943 Da. Monoisotopic mass 95.953354 Da. ChemSpider ID 13835316. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.
molecular geometries . A. b. trans-1,2-dichloroethene has no dipole moment and is nonpolar, while cis-1,2-dichloroethene has a dipole moment and is polar. Q9. Iodine reacts with fluorine to yield several compounds with the molecular formula \ (IF_n\) with \ (n\) varying from 1, 3, 5, and 7. Draw the Lew structure of each. Lewis structure of C 2 H 2 Cl 2. The Lewis structure of C2H2Cl2 contains one double bond and four single bonds, with two carbons in the center, and each carbon is attached with one hydrogen and one chlorine. There are three lone pairs on each chlorine atom, and carbon atom and hydrogen atom do not have any lone pair.
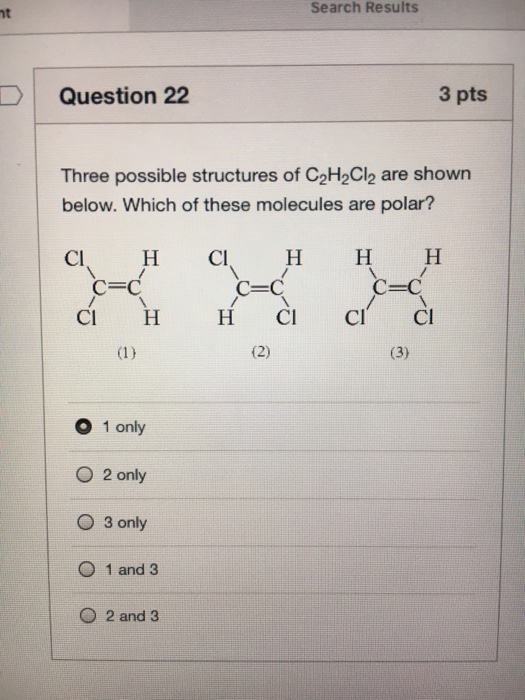
Lewis Dot Structure For C2h2cl2 Drawing Easy
Description 1, 2-Dichloroethene, also called 1, 2-dichloroethylene, is a highly flammable, colorless liquid with a sharp, harsh odor. It is used to produce solvents and in chemical mixtures. You can smell very small amounts of 1, 2-dichloroethene in air (about 17 parts of 1, 2-dichloroethene per million parts of air [17 ppm]). Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".




