Physical properties are characteristics that describe matter. They include characteristics such as size, shape, color, and mass. Many of these properties can be quantitative in nature. For example, quantitative physical properties of water would be the boiling point (100 °C / 212 °F) and melting point (0°C / 32 °F). Matter and elements The term matter refers to anything that occupies space and has mass—in other words, the "stuff" that the universe is made of. All matter is made up of substances called elements, which have specific chemical and physical properties and cannot be broken down into other substances through ordinary chemical reactions.
Properties Of Matter Chart Sorting Activity Tpt Gambaran
H igh energy state where atoms can lose an electron and both species coexist. Most of the matter of the universe is in the plasma state. The following video gives a good description of the plasma state. Video 1A.3. 1 1 A .3. 1: YouTube Video (3:32 min): "Plasma, The Most Common Phase of Matter in the Universe", uploaded by SciShow ( https. List of states of matter Matter organizes into various phases or states of matter depending on its constituents and external factors like pressure and temperature. In common temperatures and pressures, atoms form the three classical states of matter: solid, liquid and gas. Matter is a general term describing any 'physical substance'. By contrast, mass is not a substance but rather a quantitative property of matter and other substances or systems; various types of mass are defined within physics - including but not limited to rest mass, inertial mass, relativistic mass, mass-energy . A liquid flows and takes the shape of its container, except that it forms a flat or slightly curved upper surface when acted upon by gravity. (In zero gravity, liquids assume a spherical shape.) Both liquid and solid samples have volumes that are very nearly independent of pressure. A gas takes both the shape and volume of its container.
Classification of matter General Science Quiz Quizizz
Course: Chemistry library > Unit 11. Lesson 1: States of matter. States of matter. States of matter follow-up. Specific heat and latent heat of fusion and vaporization. Specific heat, heat of fusion and vaporization example. Chilling water problem. Change of state example. Vapor pressure. Phases of Matter and Phase Diagrams. A phase diagram is a graphical representation of pressure and temperature of a material. Phase diagrams show the state of matter at a given pressure and temperature. They show the boundaries between phases and the processes that occur when the pressure and/or temperature is changed to cross these boundaries. 1: Introduction - Matter and Measurement 1.3: Classification of Matter 1.2: States of Matter 1.4: Chemical Elements and Symbols Learning Objectives Categorize different types of matter as a pure substances or mixtures. Explain the difference between an element and a compound. Explain the difference between a homogeneous mixture and a heterogeneous mixture.
Ch. 2 classification of matter ppt
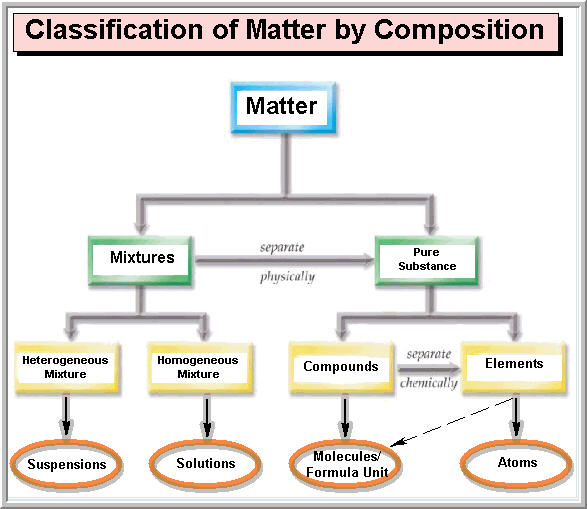
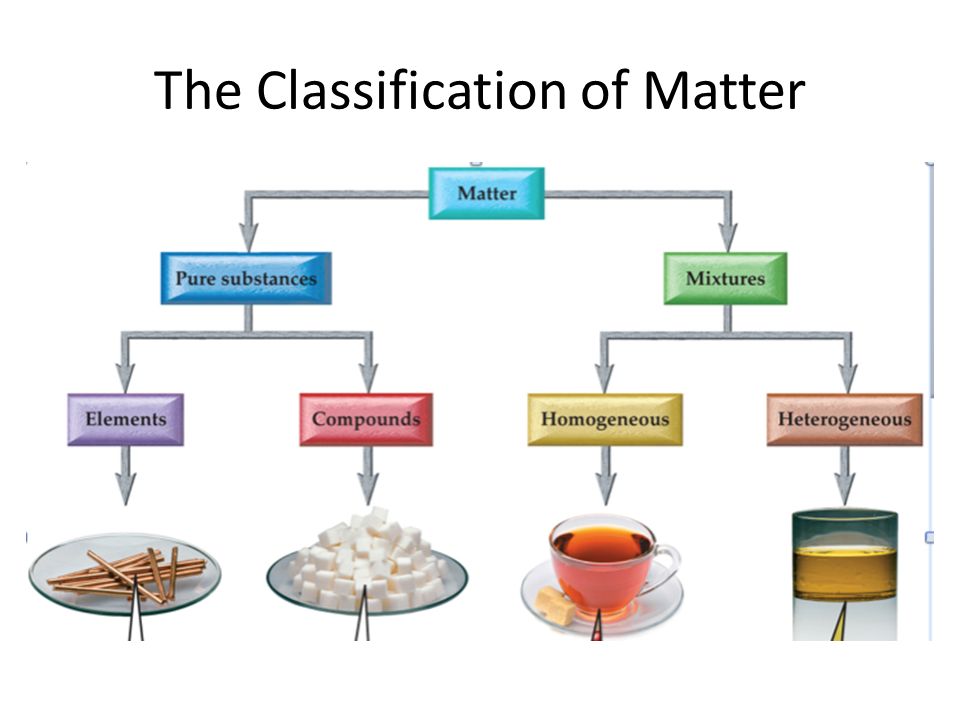
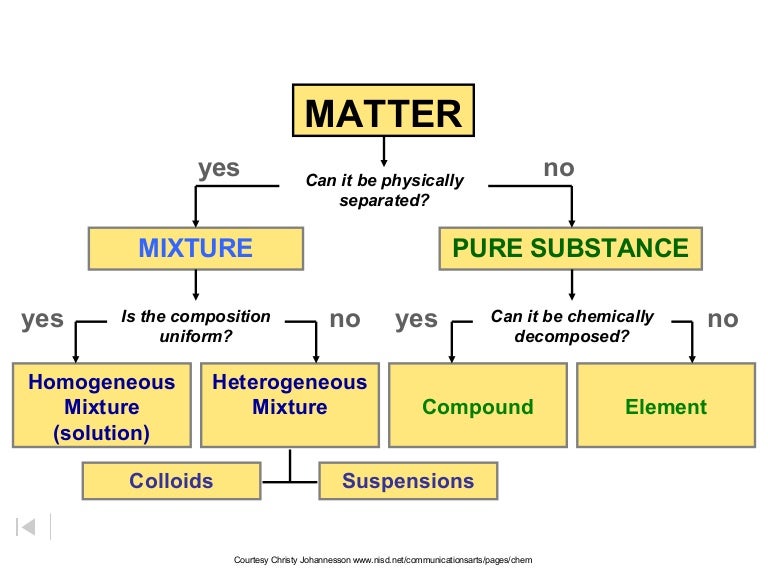
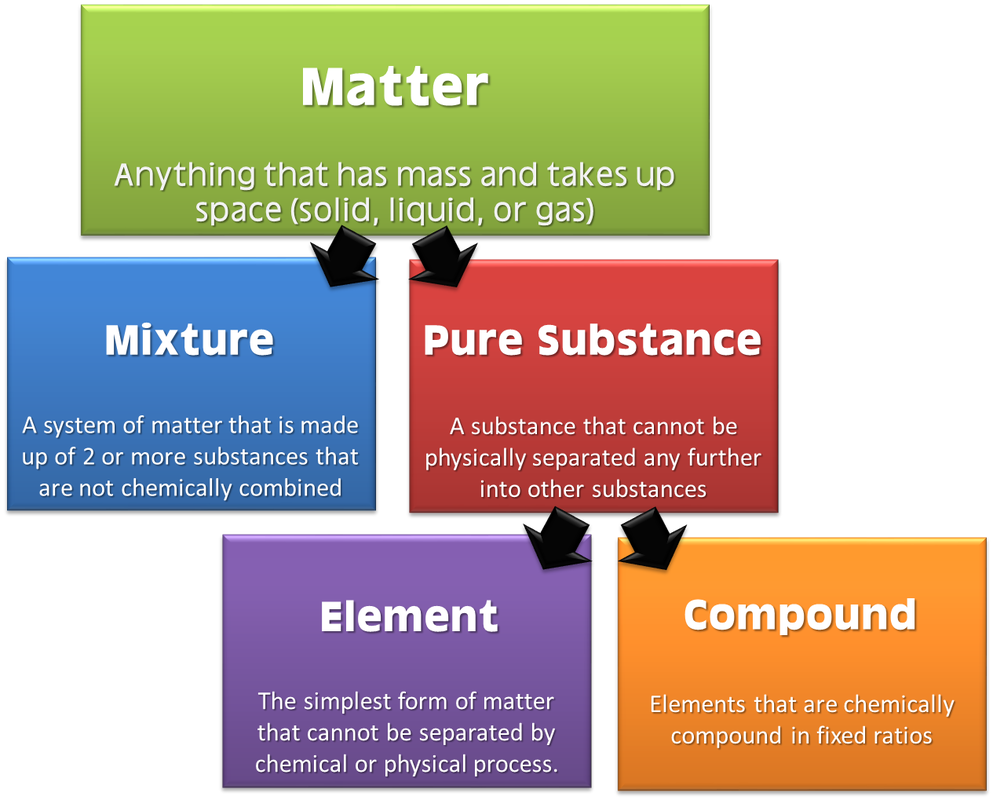
The four main states of matter are solids, liquids, gases, and plasma. Under exceptional conditions, other states of matter also exist. A solid has a definite shape and volume. A liquid has a definite volume, but takes the shape of its container. A gas lacks either a defined shape or volume. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Mixtures are physical combinations of two or more elements and/or compounds. Mixtures can be classified as homogeneous or heterogeneous. Elements and compounds are both examples of pure substances.
A matter flowchart is a diagram usually used widely in chemistry to classify matter. The matter will be categorized into two types: a mixture and pure substances in this chart. There is a homogeneous (solution) or heterogeneous mixture in the mixture section, while compound and element are what are in the pure substance section. Purposes The four natural states of matter are: Solids, liquids, gases and plasma. Bose-Einstein condensate s, however, are only made in the lab. Other exotic states of matter can also be manufactured.
MATTER CLASSIFICATION, PROPERTIES AND CHANGES
In the three years since the Jan. 6, 2021, assault on the U.S. Capitol, federal prosecutors have charged more than 1,265 defendants across nearly all 50 states and D.C. and secured sentences of. This stack includes visually appealing charts with definitions and properties of the three states of matter. Tackling one at a time, work your way through amazing activity formats like cut and glue activities, picture and word sorting, fill up and many more to build knowledge and test comprehension. Try some of these worksheets for free!




