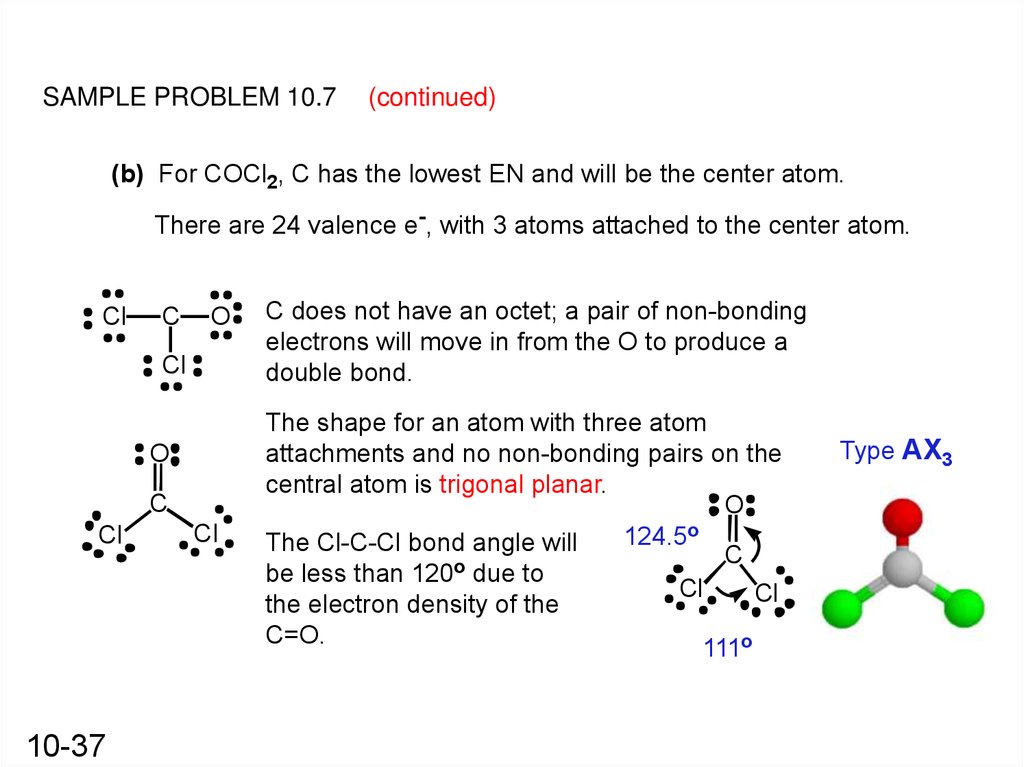
COCl2 molecule consists of one C, one O, and Cl atoms. The total number of valence electrons = 4 + 6 + 7*2 = 10 + 14 = 24. Step 2: Now, we will have to find out the element which will take up the position of the central atom. COCl2 (Phosgene) Molecular Geometry, Bond Angles (and Electron Geometry) Wayne Breslyn 726K subscribers Join Subscribe Subscribed 21K views 2 years ago An explanation of the molecular.

COCl2 (Phosgene) Molecular Geometry, Bond Angles (and Electron Geometry
It is an inorganic compound that comprises Cobalt and Chlorine atoms. CoCl2 is a crystalline solid that is sky-blue in color. It is readily soluble in water, alcohol, and acetone. It occurs at different levels of hydration as dehydrates and hexahydrates. These versions of the salt are purple and pink, respectively. A step-by-step explanation of how to draw the COCl2 Lewis Dot Structure (Phosgene).For the COCl2 structure use the periodic table to find the total number of. Structure and basic properties Phosgene is a planar molecule as predicted by VSEPR theory. The C=O distance is 1.18 Å, the C−Cl distance is 1.74 Å and the Cl−C−Cl angle is 111.8°. [9] Phosgene is a carbon oxohalide and it can be considered one of the simplest acyl chlorides, being formally derived from carbonic acid . Production The crystal unit of the solid hexahydrate CoCl 2 •6 H 2O contains the neutral molecule trans - CoCl 2(H 2O) 4 and two molecules of water of crystallization. [8] This species dissolves readily in water and alcohol . The anhydrous salt is hygroscopic and the hexahydrate is deliquescent. [citation needed]
PPT Ch. 9 and 10 Chemical Bonding PowerPoint Presentation, free
Hi Guys!COCl2 is a chemical formula for a Phosgene molecule. It comprises one Carbon, one Oxygen, and two Chlorine atoms. In this video, we share a step-by-s. What is the molecular shape of COCl2? The C - Cl bond in COCl2 is polar or nonpolar? What is the Cl - C - Cl bond angle? The molecule COCl2 is polar or nonpolar? This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer COCl2 Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles. In the COCl 2 Lewis structure, there are two single bonds and one double bond around the carbon atom, with two chlorine atoms and one oxygen atom attached to it. Two chlorine atoms with single bonds have three lone pairs, and one oxygen atom with a single bond has two lone pairs. COCl2 Lewis Structure - How to Draw the Lewis Structure for COCl2

a Structural descriptions of an optimized free phosgene (COCl2
Molecular Formula Cl Co Average mass 129.839 Da Monoisotopic mass 128.870911 Da ChemSpider ID 22708 - Charge More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users Cobalt (II) chloride [Wiki] 231-589-4 [EINECS] 7646-79-9 [RN] The molecular shape of COCl 2 is trigonal planar, or AX 3 using Valence Shell Electron Pair Repulsion (VSEPR) theory. Hence, the molecular geometry of COCl 2 only has 120 degree bond angles in the molecule. COCl 2 looks like this: How do you find the molecular geometry of COCl2?
What is this molecule? COCl 2 is the chemical formula for carbonyl chloride, also known as phosgene. It is a colorless gas at room temperature with a pungent odor. Phosgene is a highly toxic compound that was historically used as a chemical warfare agent during World War I. In the Lewis structure for COCl 2 there are a total of 24 valence electrons. You'll need to form a double bond between the Carbon and Oxygen to complete the octet on the Carbon atom. Transcript: Let's do the Lewis structure for COCl2. Carbon, in group 4 or 14, has 4 valence electrons. Oxygen in group 6, sometimes called 16, 6 valence electrons.
The Shapes of Molecules презентация онлайн
Steps of drawing COCl2 lewis structure Step 1: Find the total valence electrons in COCl2 molecule. In order to find the total valence electrons in a COCl2 molecule, first of all you should know the valence electrons present in carbon atom, oxygen atom as well as chlorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.) 3a. Drawing the structure of CoCl2 Table view List view 3a. Drawing the structure of CoCl2 COCI Lone pairs of electrons (central atom) Bonding groups (central atom) Total valence electrons VSEPR Molecular shape (central atom) Choose Choose. 3b. Evaluating the structure of CoCl2 Table view List view 3b.




