A step-by-step explanation of how to draw the IF2- Lewis Structure. For the IF2- structure use the periodic table to find the total number of valence electrons for.more.more A. IF2- lewis structure contains one iodine atom at the middle position whereas two fluorine atoms at the surrounding position. There are three lone pairs present on the central atom of IF2- lewis structure. Also, the iodine central atom in IF2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell.

IF2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
How to Draw the Lewis Dot Structure for IF2 + Wayne Breslyn 697K subscribers Subscribe 2.6K views 1 year ago A step-by-step explanation of how to draw the IF2 + Lewis Dot Structure. For the. In if2- lewis structure we see that each F atom has 8 valance electrons and completes their octet. In I forms 2 I-F bonds and each bond contain 2 electrons. There is also 3 lone pairs that present on I atom making a total of 10 electrons around I atom. IF2- Lewis Structure | How to Draw the Lewis Structure for IF2- - YouTube © 2023 Google LLC IF2- comprises one Iodine and two Fluorine atoms. The negative charge on this ion indicates. An explanation of the molecular geometry for the IF2 - ion (Iodine difluoride anion) including a description of the IF2 - bond angles. The electron geometry.

[Solved] Drew the lewis diagram, Determine the molecular geometry and
The IF 2- Lewis structure you'll need to put more than eight valence electrons on the Iodine atome. In the Lewis structure for IF 2- there are a total of 22 valence electrons. Watch on See the Big List of Lewis Structures Transcript: This is the IF2- Lewis structure. Iodine, 7 valence electrons. In IF 2- Lewis structure, there are two single bonds around the iodine atom, with two fluorine atoms attached to it, and each atom has three lone pairs. Also, there is a negative (-1) charge on the iodine atom. IF2- Lewis Structure: How to Draw the Lewis Structure for IF2 - Watch on Contents Steps #1 Draw a rough skeleton structure Lewis structure of IF2- ion contains two single bonds between the Iodine (I) atom and each Fluorine (F) atom. The Iodine atom (I) is at the center and it is surrounded by 2 Fluorine atoms (F). The Iodine atom as well as both the Fluorine atoms have 3 lone pairs. The Iodine atom has -1 formal charge. IF2- lewis structure has an Iodine atom (I) at the center which is surrounded by two Fluorine atoms (F). There is a single bond between the Iodine atom (I) and each Fluorine atom (F). There is a -1 formal charge on the Iodine atom (I).

IF2 Lewis structure, Molecular geometry, and Polar or nonpolar
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as. The Lewis structure of H 2 O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two chemical bonds: We predict that these four regions are arranged in a tetrahedral fashion (Figure 7.23), as indicated in Figure 7.19. Thus, the electron-pair geometry is tetrahedral and the molecular.
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 6.4.1 6.4. 1 shows the Lewis symbols for the elements of the third period of the periodic table. Electron dots are typically arranged in four pairs located on the four "sides" of the atomic symbol. Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
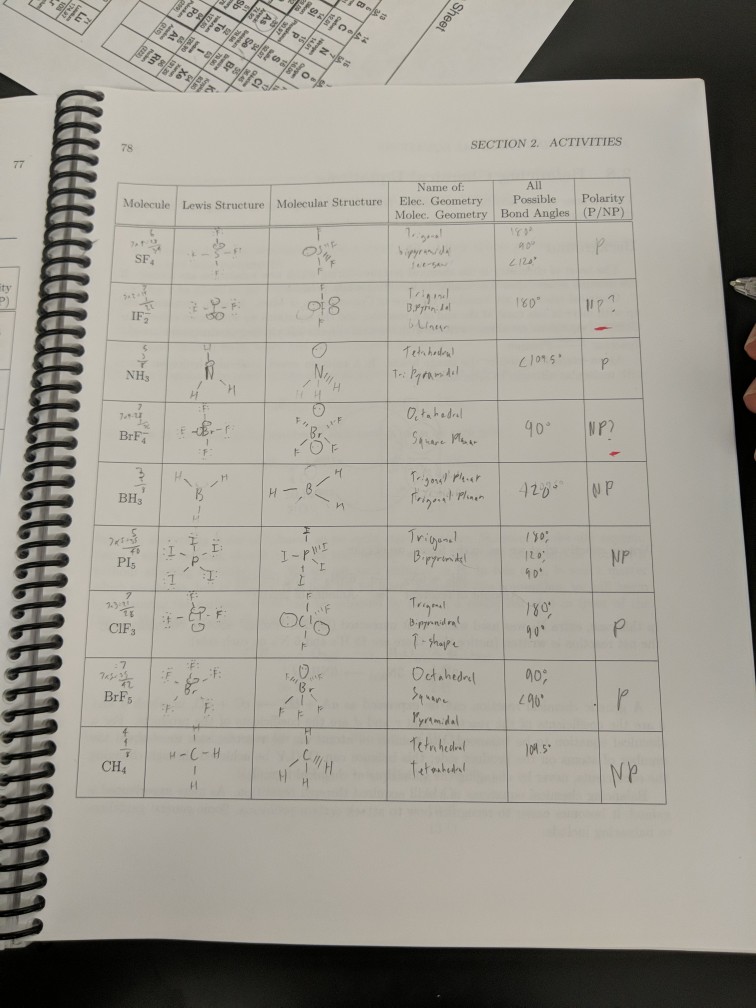
Solved SECTION 2. ACTIVITIES Molecule Lewis Structure
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of. IF2- comprises one Iodine and two Fluorine atoms. The negative charge on this ion indicates that it accepts an additional electron to form this structure. Generally, the ions that accept electrons acquire a negative charge, whereas those that donate electrons acquire a positive charge.



