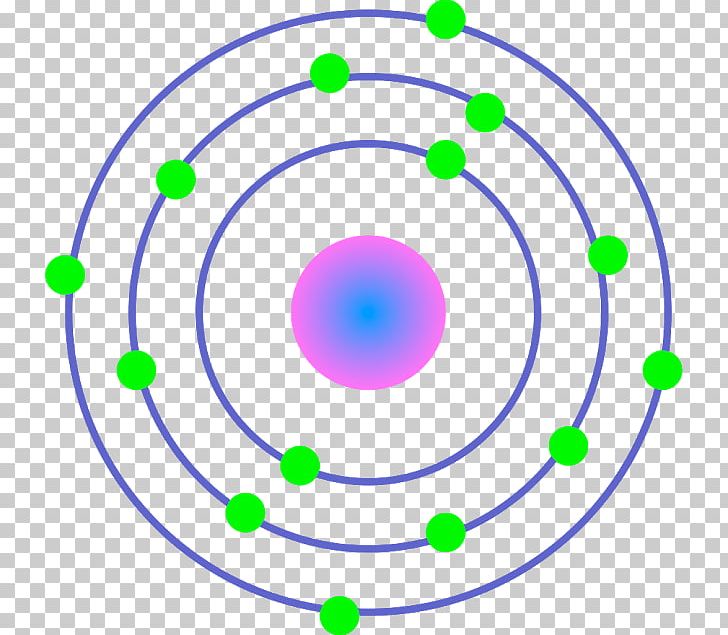
Iron Bohr model December 14, 2023 by Deep The information on this page is fact-checked. Iron Bohr model In the iron Bohr model, the nucleus comprises 26 protons and 30 neutrons. Surrounding this nucleus are four electron shells, accommodating a total of 26 electrons. The electron cloud model An electron cloud model of a helium-4 atom is shown below. [What do the scales mean on this model?] In this model, the black "cloud" represents the volume of space where electrons are likely to be found. The darker the region, the more likely electrons are to be found there.

Iron(III) Chloride AMERICAN ELEMENTS ® / Ferric Chloride
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is. Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.. The image below shows the emission spectrum of iron. Because each element has a unique emission spectrum, elements can be defined using them. Figure \(\PageIndex{3}\): Atomic. The Bohr Model for Hydrogen (and other one-electron systems) In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum.. iron, and carbon, along with smaller amounts of other elements. During the solar eclipse of 1868, the. Figure \(\PageIndex{7}\) In Bohr's Model of the atom, electrons absorb energy to move to a higher level and release energy to move to lower levels. (CC BY-SA 3.0; Kurzon). The evidence used to support Bohr's model came from the atomic spectra. He suggested that an atomic spectrum is made by the electrons in an atom moving energy levels.

Iron (Fe) Bohr Model
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted. The Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (hν). The orbits in which the electron may travel are shown as grey circles; their. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that Bohr's model was taken seriously, despite the many assumptions that Bohr needed to derive it. Figure \(\PageIndex{1}\): Quantum numbers and energy levels in a hydrogen atom. Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately dropping back to a lower energy level and emitting the energy difference between the two energy levels. The existence of the atomic spectra is support for Bohr's model of the atom.
Bohr Model Model Atomic Iron Atomic Orbital PNG, Clipart, Area, Atom
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to. The Bohr Model of Iron (Fe) has a nucleus that contains 30 neutrons and 26 protons. This nucleus is surrounded by four electron shells namely K-shell, L-shell, M-shell, and N-shell. The 1 st shell has 2 electrons, the 2 nd shell has 8 electrons, the 3 rd shell has 14 electrons and the 4 th shell has 2 electrons. Page Contents show
The Bohr model, introduced by Danish physicist Niels Bohr in 1913, was a key step on the journey to understand atoms. Ancient Greek thinkers already believed that matter was composed of tiny. Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?

Iron atom Bohr model stock vector. Illustration of isolated 267662069
The Bohr model shows that the electrons in atoms are in orbits of differing energy around the nucleus (think of planets orbiting around the sun). Bohr used the term energy levels (or shells) to describe these orbits of differing energy. He said that the energy of an electron is quantized, meaning electrons can have one energy level or another. The Bohr model describes the structure of an atom as a central nucleus containing protons and neutrons, with electrons orbiting in specific energy levels around it. Electrons can jump between these energy levels by absorbing or emitting energy. This model helps us understand basic atomic properties and electron behavior in atoms. K Shell




