Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. As a result, the dipole moment of Acetone is around 2.69 D. Acetone exists in a liquid state at room temperature. It is colorless in appearance and has a characteristic odor. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than alcohols. Then again, acetone (and other carbonyl containing solvents) are, indeed, poor solvents when using strong bases due to their relatively high acidity.
MakeTheBrainHappy Is Acetone Polar or Nonpolar?
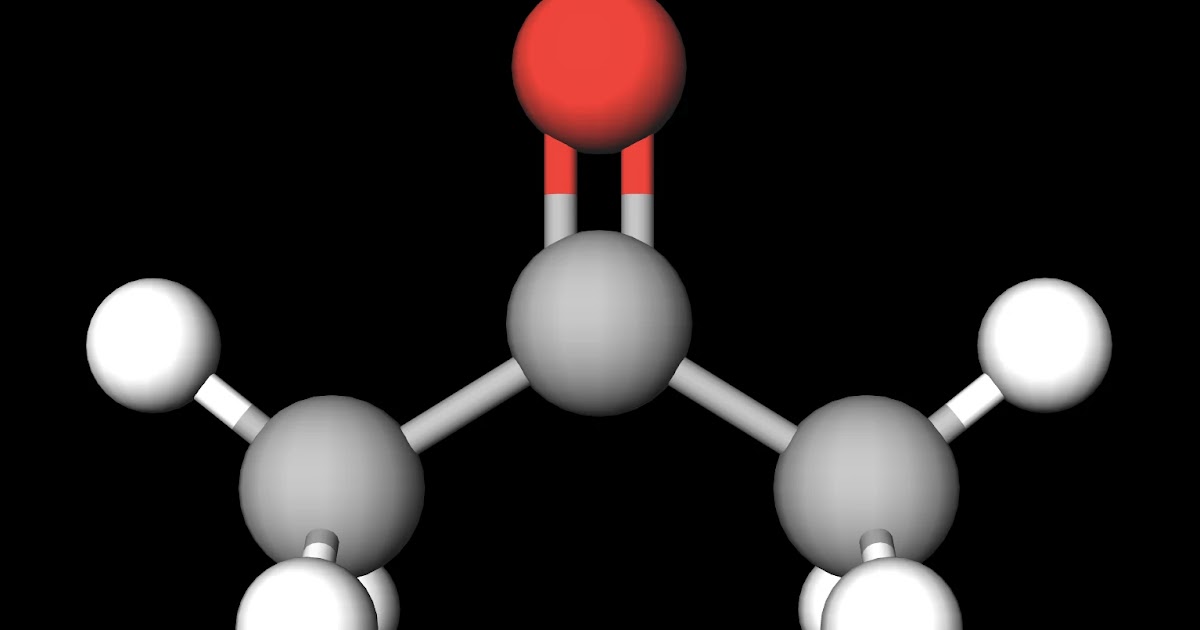
Acetone ( 2-propanone or dimethyl ketone) is an organic compound with the formula (CH3)2CO. [22] It is the simplest and smallest ketone ( >C=O ). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. To determine if C3H6O (Acetone) is a polar or non-polar molecule we need to look at the Lewis structure, molecular geometry, and the electronegativity of the atoms in C3H6O..more.more. Acetone (C3H6O) is a POLAR molecule because the Oxygen (O) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire acetone molecule polar. Acetone molecules are polar because of the positive and negative charges formed by their carbonyl group. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds.

Is Acetone Polar Nonpolar Or Ionic girtbir Coub
Category: Science & Tech Also called: 2-propanone Or: dimethyl ketone Related Topics: ketone cordite On the Web: Australian Government - Department of Climate Change, Energy, the Environment and Water - Acetone (Dec. 29, 2023) (Show more) See all related content → Acetone is used as an industrial solvent and is a key component in the production of plexiglass. In the latter, acetone is used as an intermediate. Acetone is converted to acetone cyanohydrin, which is further synthesized to obtain methyl methacrylate (plexiglass). Acetone is also used medically as a solvent and an anti-convulsing agent. Acetone contains a polar C=O double bond oriented at about 120° to two methyl groups with nonpolar C-H bonds. The C-O bond dipole therefore corresponds to the molecular dipole, which should result in both a rather large dipole moment and a high boiling point. Thus we predict the following order of boiling points: Acetone is a small molecule that has very non-polar and polar properties simultaneously. Its polar C=O bond makes it miscible (soluble) in water , while its non-polar methyl (CH 3 ) groups can interact with non-polar compounds.

Polar acetone 1 Liter Pletfjerner
Acetone is a polar solvent, but has non-polar properties. It can remove, dissolve, and clean non-polar substances and stains, such as wax and tree sap, due to its 2 non-polar bonds. Is acetone polar or nonpolar? Because of the carbonyl group, acetone is a somewhat polar molecule. There are different degrees of polarity, and acetone is less polar than water, because only part of the acetone molecule has a polar bond. More examples of polar molecules.
So acetone is a polar aprotic solvent. Some other polar aprotic solvents are listed below. Answer link. Acetone is a polar aprotic solvent. A solvent is polar if it has a dipole moment greater than 1.6 D and a dielectric constant greater than 5. The values for acetone are µ = 2.88 D and ε = 21. So acetone is a polar solvent. 0.447 g/100 mL. 4.7 mL (gas)/ 100 mL. 6.5 mL (gas)/ 100 mL. 8.0 g/100 mL. This table shows that alcohols (in red) have higher boiling points and greater solubility in H haloalkanes and alkanes with the same number of carbons. It also shows that the boiling point of alcohols increase with the number of carbon atoms.

Is acetone polar or nonpolar? Will NaCl dissolve in it? YouTube
Take acetone (C 3 H 6 O) for example. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. This is an aprotic solvent because the highly electronegative atom, oxygen, is not bonded to a hydrogen atom. The hydrogen atoms are bonded to the carbon instead, which is a. Water Acetic Acid Ethylene Glycol Methanol Ethanol Isopropanol Pyridine Acetonitrile Nitromethane Diethylamine Aniline Dimethylsulfoxide Ethyl Acetate Dioxane Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethyl Ether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum ether Hex.




