H H ** ** ** H : C : C : O : H ** ** ** H H Q3. Give the electron pair and molecular geometries for each central atom. Q4. Give the bond polarity for each type of bond in the molecule. = There are two types of bonds in ethanol. The C-H bonds are nonpolar covalent. Q5. Is your molecule polar or nonpolar ? = Ethanol is both polar and nonpolar. Q6. Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvent Polarity. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct.
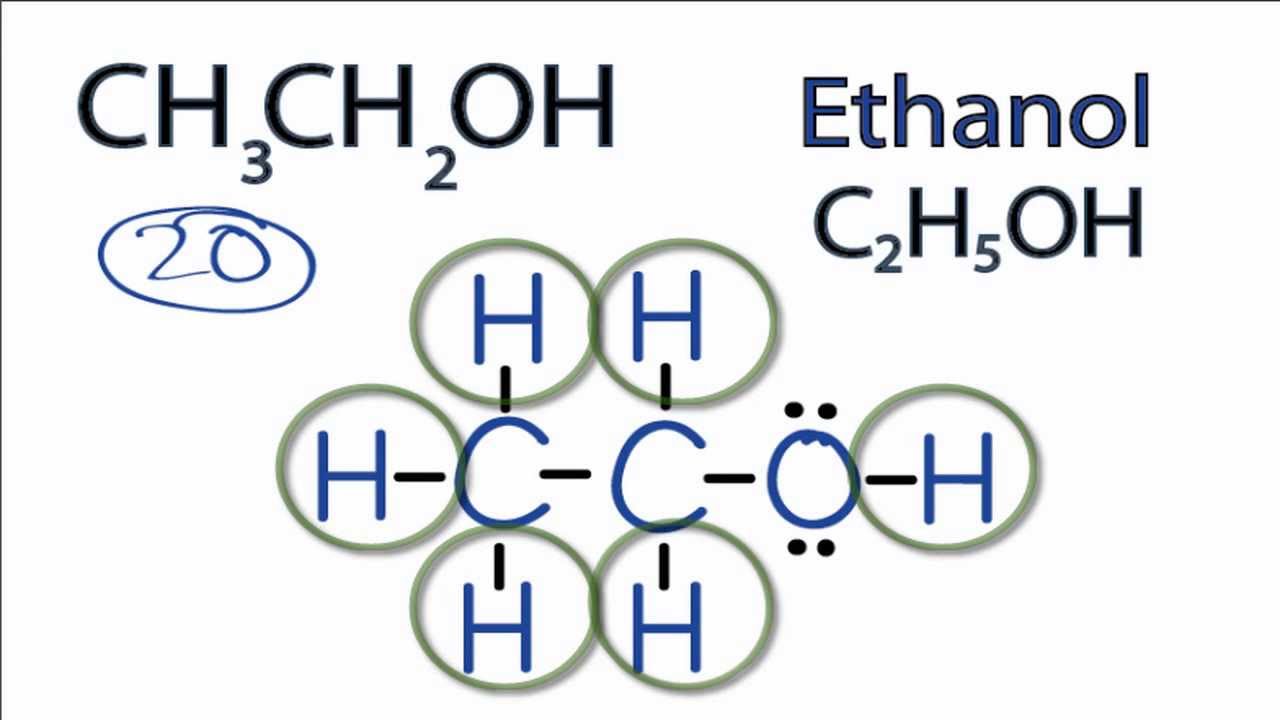
Lewis Structure For C2h5oh
CH 3 CH 2 OH represents the chemical formula for ethanol, aka ethyl alcohol, a primary alcohol commonly found in alcoholic beverages, cosmetics, perfumes, and spirits. Ethyl alcohol is a clear, colorless liquid (molar mass = 46.07 g/mol) often used as a solvent for various industrial applications. Calculate the molecular polarity (polar, non-polar) of a chemical bond based on the electronegativity of the elements. First Element Second Element Calculate Bond Polarity How To Calculate Bond Type and Polarity The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. There are 4 steps to solve this one. Expert-verified Step 1 The compound CH A 3 CH A 2 CH A 2 OH, also known as propanol or 1-propanol, is a polar covalent molecule. To determine its. View the full answer Step 2 Unlock Step 3 Unlock Step 4 Unlock Answer Unlock Previous question Next question Not the question you're looking for? Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer Ethanol molecule has an alkyl chain, and the O-H group is attached at the end of the.

Solved Name Lab Partner Lewis Structure (ethanol,
Water is considered a polar solvent. Which substances should dissolve in water? methanol (CH 3 OH) sodium sulfate (Na 2 SO 4) octane (C 8 H 18), a non-polar organic compound; Solution. Because water is polar, substances that are polar or ionic will dissolve in it. Because of the OH group in methanol, we expect its molecules to be polar. Exercise 2.12: Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar, hydrophobic 'solvent'. Decide on a classification for each of the vitamins shown below. Exercise 2.13: Both aniline and phenol are insoluble in pure water. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid. Ethanol $(\ce{CH3CH2OH})$ Propane $(\ce{CH3CH2CH3})$. When my teacher takes this question up, he said that propane is non polar, which means it only interacts via london forces, which means it has the lowest boiling points out of these three. But how is propane non polar though, when I asked, he said "I don't have an answer for this, life is. 5. "Borderline" Polar Aprotic Solvents Have Small Dipole Moments And Low (<10) Dielectric Constants. These solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Since they have intermediate polarity they are good "general purpose" solvents for a wide range of reactions.
SOLVED Draw the bondline formulas for the following compounds
Learn to determine if CH3OH (Methanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur. Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.
The boiling point of Methanol ( CH3OH) is 64.7 °C. The melting point of Methanol is -97.6 °C. The molecular weight of Methanol is 32.04 g/mol. It is a polar solvent and is also known as wood alcohol because it was once produced by the distillation of wood. The smell of this compound is on the sweeter side as compared to ethanol. yes Is CH3I polar or nonpolar? non polar Is KrCl2 polar or nonpolar? It's ionic, not polar Is PO4 3- polar or nonpolar? It is non polar

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education
Table of Contents [ hide] 1 Is CH3CH2OH polar or nonpolar? 2 Is CH2CL2 polar? 3 Which is more polar CCl4 or CH2Cl2? 4 Is ethane a polar or nonpolar molecule? 5 Is the word c2f2 polar or nonpolar? 6 What is the difference between polar and non polar molecules? Is CH3CH2OH polar or nonpolar? Because CO is a polar molecule, it experiences dipole-dipole attractions. Because N 2 is nonpolar, its molecules cannot exhibit dipole-dipole attractions. The dipole-dipole attractions between CO molecules are comparably stronger than the dispersion forces between nonpolar N 2 molecules, so CO is expected to have the higher boiling point.




