SeO3 is a NONPOLAR molecule because all the three bonds (Se=O bonds) are identical and SeO3 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SeO3 lewis structure and its 3D geometry. Why is SeO3 a Nonpolar molecule? (Explained in 3 Steps) In summary, SeO3 is a nonpolar molecule due to its trigonal planar molecular geometry, which results in the cancellation of bond dipoles. Understanding the properties of SeO3, such as its nonpolarity, is crucial in various applications, including its use as a reagent in organic synthesis and as an oxidizing agent in chemical reactions.
Is Sulfur Trioxide (SO3) Polar or NonPolar? Lewis Structure (The
Selenium trioxide is the inorganic compound with the formula Se O 3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se (VI) compounds. [4] Preparation Selenium trioxide is difficult to prepare because it is unstable with respect to the dioxide: 2 SeO 3 → 2 SeO 2 + O 2 Molecules with dipoles (polar molecules) contain polar bonds. lewis dot structure, molecular shape, atom electronegativity. is ClF2 polar or nonpolar. polar. is SeO3 polar or nonpolar. nonpolar. Hydrogen bonding. An unusually large dipole-dipole interaction, H "bonds" to a lone pair (on N, O, or F) only ones it can bond to. Science Chemistry Chemistry questions and answers For the following compound: SeO32- Draw the Lewis Structure Determine the geometry Determine whether the compound is polar or non-polar This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer It also discusses if SeO3 is polar or nonpolar in addition to the bond angle, hybridization, and the molecular geometry. This video shows you how to draw the lewis dot structure of SeO3 also known as selenium trioxide. It also discusses if SeO3 is polar or nonpolar in addition to the bond angle, hybridization, and the molecular geometry..

Is SO3 Polar or Nonpolar? Techiescientist
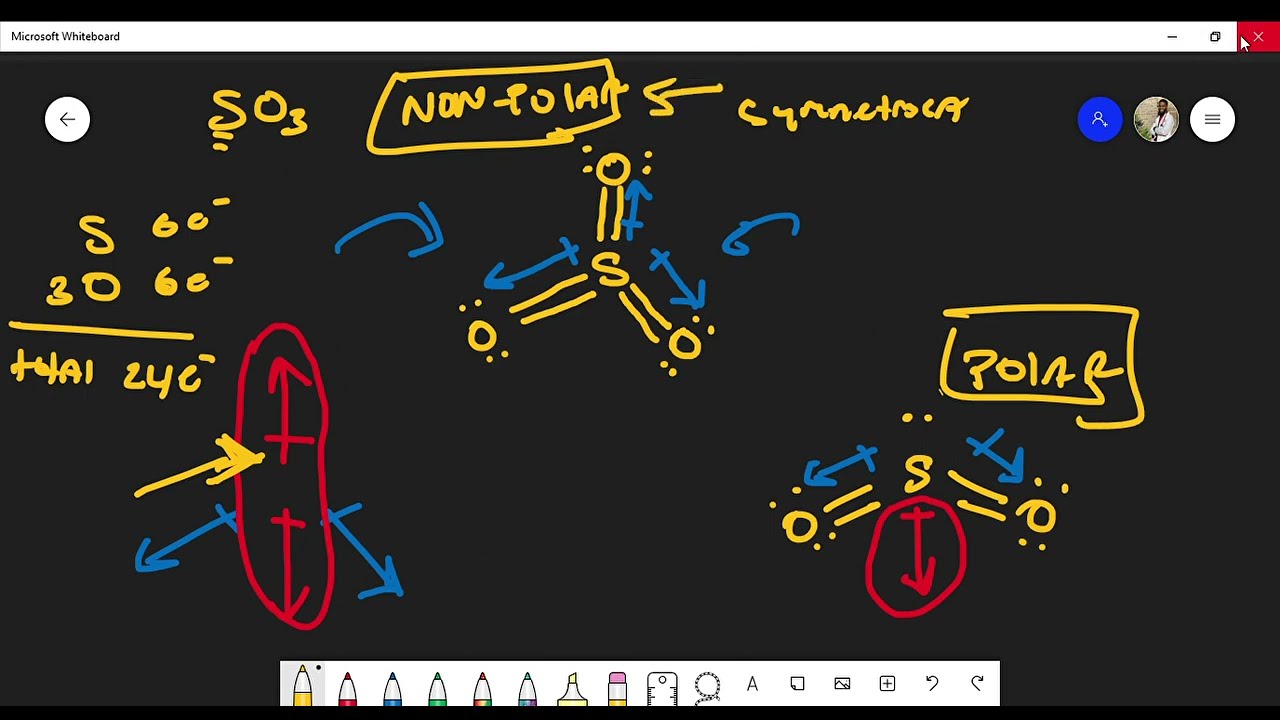
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms. 1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances. Figure \(\PageIndex{14}\): (a) Molecules are always randomly distributed in the liquid state in the absence of an electric field. (b) When an electric field is applied, polar molecules like HF will align to the. Differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. Nonpolar covalent bonds have an equal distribution of electron density between the two nuclei. Polar covalent bonds have an unequal distribution of electron density with the more electronegative atom having greater electron.

Is CS2 polar or nonpolar? YouTube
1 year, 1 month ago Georgia Institute of Technology We don't have your requested question, but here is a suggested video that might help. Classily each molecule as polar or nonpolar: Polur Nonpolar Ansat Bant You must be signed in to discuss. Numerade has step-by-step video solutions, matched directly to more than +2,000 textbooks. H2SeO3 ⇌ SeO2 + H2O Let us study its lewis structure, geometry, and hybridization. Contents show Lewis Structure of Selenium Dioxide (SeO2) Also called the Lewis dot structure, it is a pictorial representation of the behavior and arrangement of valence electrons within an atom. It uses dots to represent valence electrons and lines to show bonds.
Is SeO3 polar or nonpolar ? Question = Is SeO3 polar or nonpolar ? Answer = SeO3 ( Selenium trioxide ) is Nonpolar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Sulfur brings 6, and oxygen brings 3 each. That means; SO3 has 24 valence electrons. 6 + (3 x 6) = 24. Now have a look of Lewis Structure again; When we draw it, firstly we get the three structures at the top. Sulfur in the center and Oxygen around it is making a connection (each) to the central atom. There should be single bonds initially.

Ch4 Polar Or Nonpolar / Solution Is The Ch4 A Polar Or Non Polar Chemistry
But why? And how can you say that SeO2 is a polar molecule? Want to know the reason? Let's dive into it! SeO2 is a POLAR molecule because the Oxygen (O) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. If you look at the Lewis structure for SO3 it appears to be a symmetrical molecule. However, to determine if SO3 is polar we need to look at the molecular.




