If you look at the Lewis structure for SO3 it appears to be a symmetrical molecule. However, to determine if SO3 is polar we need to look at the molecular. Sulfur Trioxide comprises one Sulfur and three Oxygen atoms. To determine its polarity, we will first look at its Lewis Structure to see the atoms' arrangeme.

Best Overview Is SO3 Polar or Nonpolar? Science Education and Tutorials
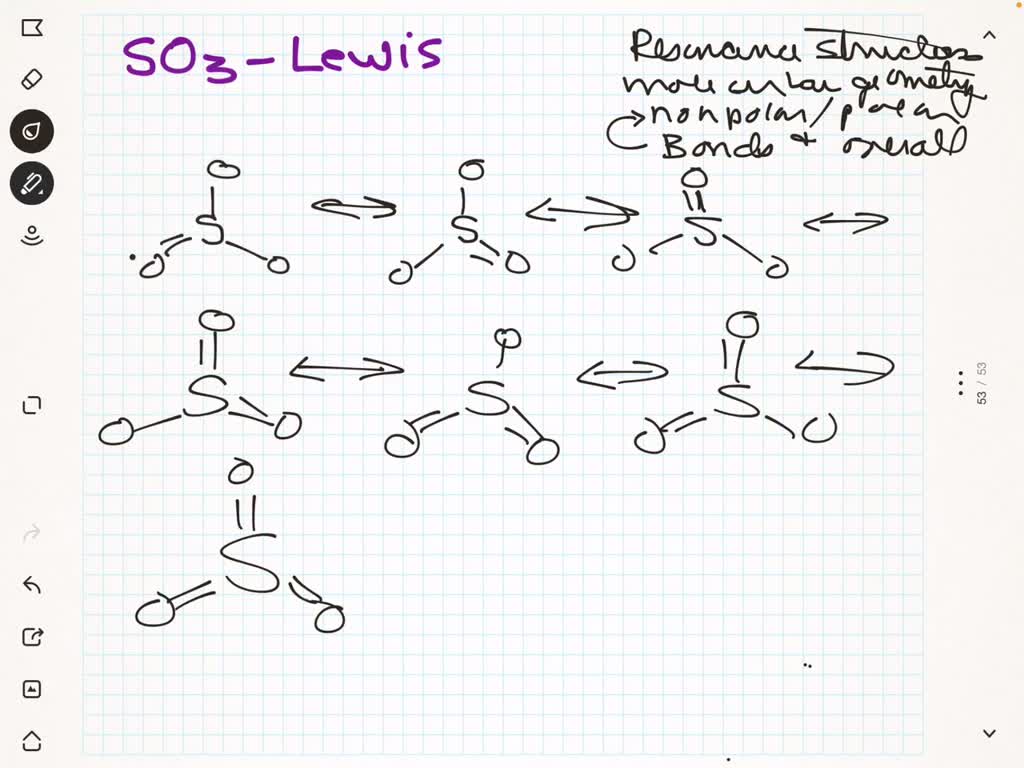
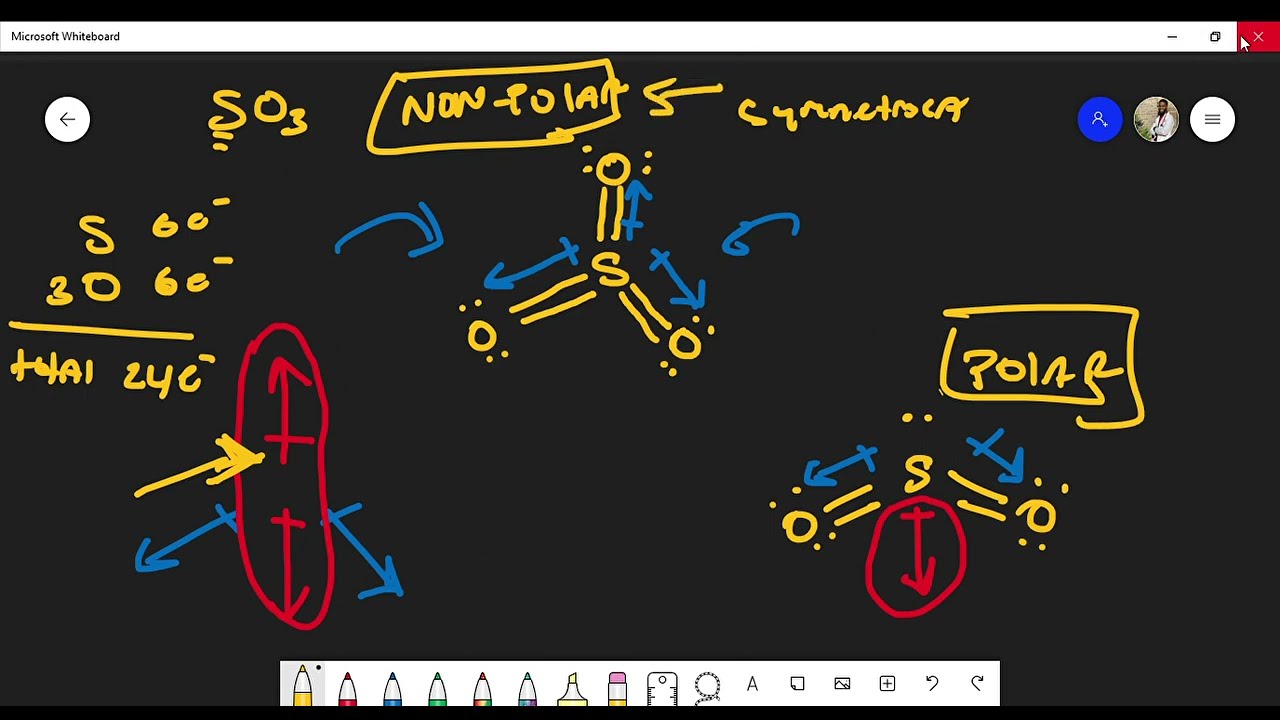
SO3 (Sulfur Trioxide) has a trigonal planar structure. It consists of one Sulfur atom surrounded by 3 Oxygen atoms symmetrically. The atomic number of oxygen is 8, as a result, there are 6 electrons in the vacant shell of oxygen. Similarly, the atomic number of sulfur is 16 and there are 6 electrons in the vacant shell of Sulfur as well. SO3 is a NONPOLAR molecule because all the three bonds (S=O bonds) are identical and SO3 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SO3 lewis structure and its 3D geometry. Why is SO3 a Nonpolar molecule? (Explained in 3 Steps) The molecule SO 3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D 3h point group. The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). [7] The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to Drawing the Lewis Structure of SO3
SOLVED a. Give the Lewis electron dot structure for SO3. If there are
SO3 which is also spelled as Sulphur Trioxide sometimes, is a trigonal planar molecule that is non-flammable. In this article, I will provide you some information regarding SO3 molecular geometry with the explanations of Lewis structure, polarity, and hybridization. Contents Sulfur Trioxide Molecular Geometry Lewis Structure of SO3 Polarity of SO3 SO3, so3 polar or nonpolar / By SciEduTut / is so3 polar, is so3 polar or nonpolar, lewis structure for so3, polarity of so3, so3 charge, so3 lewis structure, so3 lewis structure polar or nonpolar, so3 molar mass, so3 molecular geometry, so3 polar, so3 polar or nonpolar, so3 polarity, sulfur trioxide lewis structure DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-e.Snapchat: https://www.snapchat.com/add/kravonoInstagram: https://www.instagram.c. Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.

Is SO3 Polar or Nonpolar? Polarity of SO3
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based. Sulfur trioxide is a compound with the chemical formula SO3. This compound is of great importance and studied widely as it reacts with the water present in the air to produce sulfuric acid. When this sulfuric acid, in the gaseous state, mixes with the rain and falls on the Earth, it is called acid rain.
As this difference lies between 0.4 and 1.6, the bonds formed in the SO3 molecule are polar covalent bonds. However, the net charge on the sulfur trioxide molecule is zero owing to its geometry, due to which the molecule in itself is non-polar. The chemical substance sulphur trioxide (also known as nisso sulfan) has the formula SO 3 (alternative spelling: sulphur trioxide). "Unquestionably the most significant commercially", according to Sulphur Oxide. It is produced in enormous quantities as a precursor to sulphuric acid.
Is Sulfur Trioxide (SO3) Polar or NonPolar? Lewis Structure (The
Explanation 1: The sulfur trioxide (SO3) is a nonpolar molecule because, in SO3, electrons' sharing is equal, and the lewis structure of SO3 appears to be an asymmetrical molecule. Explanation 2: The sulfur trioxide (SO3) is a nonpolar molecule because the shape of the SO3 is trigonal planar. If there is only one bond in the molecule, the bond polarity determines the molecular polarity.Any diatomic molecule in which the two atoms are the same element must be a nonpolar molecule. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule where one end of the molecule is slightly positive, while the other end is slightly negative.




