Laminins are large cell-adhesive glycoproteins that are required for the formation and function of basement membranes in all animals. Structural studies by electron microscopy in the early 1980s revealed a cross-shaped molecule, which subsequently was shown to consist of three distinct polypeptide chains. The laminin-integrin interface is made up of several binding sites located on all five subunits, with the laminin γ1 chain C-terminal portion providing focal interaction using two carboxylate.

N A I T H G I N K letsgo!
Laminins are large cell-adhesive glycoproteins that are required for the formation and function of basement membranes in all animals. Structural studies by electron microscopy in the early 1980s revealed a cross-shaped molecule, which subsequently was shown to consist of three distinct polypeptide chains. Laminin is a large (900 kDa) mosaic protein composed of many distinct domains with different structures andfunctions. Globular and rodlike domains are arrangedin an extended four-armed, cruciform shape that iswell suited for mediating between distant sites on cellsand other components of the extracellular matrix. Laminins are a family of large (440-900 kDa) glycoproteins forming networks specifically found in basement membranes. Each member of the family is composed of three polypeptides, the α, β, and γ chains (Table 1 ), assembled together to form a T- or cross-shaped molecule with two or three short arms, respectively, and one long arm. Table 1. We demonstrate that our laminin system is comparable to the 3T3-J2 co-culture system in terms of basal markers' profile, colony-forming efficiency and the ability to form normal stratified.

Laminin Evidence of God's Existence? New Life ExchangeNew Life Exchange
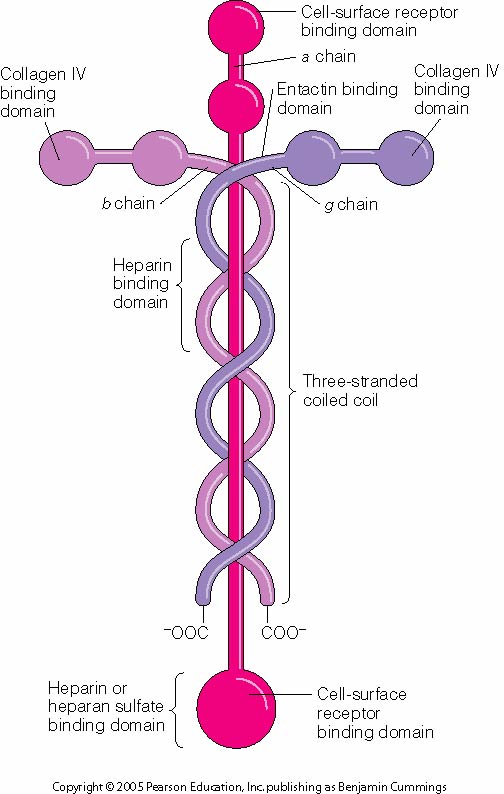
1 Altmetric Metrics Abstract Laminin, a major component of the basement membrane, plays an important role in blood brain barrier regulation. At the neurovascular unit, brain endothelial cells,. Laminin, the major non-collagenous protein of basement membranes, has been shown to be a potent stimulator of neurite outgrowth, to potentiate neuronal survival and to stimulate Schwann cell division in vitro. Under the light microscope, laminin presents a continuous expression in the normal oral mucosa or oral hyperplastic lesions [33, 34]. On the contrary, previous studies suggest a discontinuous distribution of laminin from epithelial hyperplasia to epithelial dysplasia [35,36,37,38]. The typical laminin heterotrimer is schematized as a cross-shaped structure in which the three chains wrap around each other via their laminin coiled-coil (LCC) domains to form the lower long arm of the cross (Fig. 2). Extending past this arm is a large (approximately 900 amino acid) laminin globular (LG) domain that is found at the COOH termini of all five α chains.
OC Apologist On Christ Apologetics Laminin
Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa) and possess three different chains (α, β and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition, e.g. laminin-511 contains α5, β1, and γ1 chains. [3] Laminins are a family of secreted glycoproteins that are found in many ECM formations, and like fibronectin, bind to cells via integrin receptors. The laminin protein is composed of three subunits (α, β, γ) arranged in a cruciform shape. There are multiple isoforms of each subunit yielding the variety (15) of laminin proteins catalogued to date.
Introduction The molecules of the extracellular matrix mediate interactions between embryonic cells: they are essential for cell adhesion and for cell shape, polarity and migration, and are able to induce cell differentiation and compartmentalization processes linked to morphogenesis. This basal lamina contains molecules secreted from both the presynaptic motor neuron and the postsynaptic muscle fiber and, unlike extrasynaptic muscle basement membrane, includes molecules that direct the formation of synaptic specializations ( Sanes et al ., 1978; Burden et al ., 1979 ).

17 Best images about laminin on Pinterest The cross, The very and
AFM is a good alternative to electron microscopy for determining the structures of biomolecules. One of the most distinguished features of the AFM is its ability to observe molecules in three dimensions even in physiological solu- tions, when the molecules are on flat surfaces. MATERIALS AND METHODS Reagents. Laminin-modified alginate was successfully synthesized to mimic early embryonic environment. • Adipose-derived stem cells (ADSCs) and ginsenoside Rg1 were encapsulated into laminin-alginate microspheres (ADSC-G-LAMS) by bio-electrospray method. •




