A step-by-step explanation of how to draw the CH3OH Lewis Structure.When you see a carbon with an OH attached (like CH3OH, C2H5OH, etc.) that means that he O. The Lewis Structure of a molecule gives the simplest representation of valence shell electrons around itself. Here, the valence electrons are represented by small dots and since a single bond consists of two bonding electrons, the two dots between two atoms are represented by a line instead, which represents a bond between them.

How to Draw the Lewis Structure for CH3OH (Methanol) YouTube
Lewis Dot Structure of CH3OH (Methanol) kentchemistry.com 25.1K subscribers Subscribe Subscribed 127K views 12 years ago Every Video I quickly take you through how to draw the Lewis. This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. The Lewis structure of CH3OH, also known as methanol, is a representation of the molecule's bonding and electron distribution. It provides valuable insights into the molecule's geometry, hybridization, and polarity. Let's explore the step-by-step process of determining the Lewis structure of CH3OH. Calculation of Valence Electrons 1.93K subscribers Subscribe 2K views 1 year ago Lewis Structure Hey Guys! For today's video, we are going to study the Lewis dot structure of Methanol. It has a c Lewis Structure of SO4.

Estructura De Lewis Ch3oh Compuesto
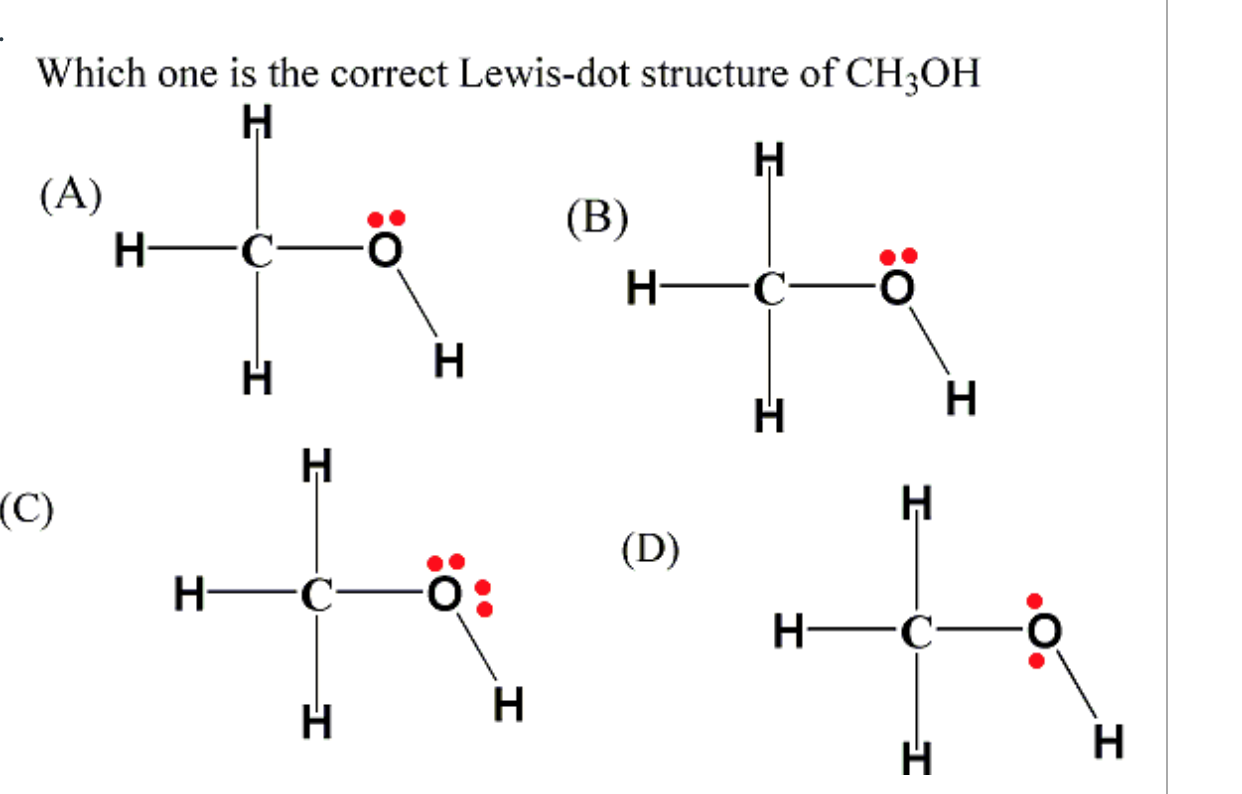
What is the Lewis structure of [//substance:CH3OH//]? Natural Language Math Input Extended Keyboard Examples Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music… May 22, 2023 by Jay Rana Ready to learn how to draw the lewis structure of CH3OH? Awesome! Here, I have explained 6 simple steps to draw the lewis dot structure of CH3OH (along with images). So, if you are ready to go with these 6 simple steps, then let's dive right into it! The molecule methanol (methyl alcohol) has the structure CH3OH, and contains fourteen valence electrons (four for carbon, six for oxygen, one each for the four hydrogens). Students should be able to construct a Lewis dot structure, making sure that the carbon and oxygen atoms have enough electrons to satisfy the octet rule (eight surrounding. CH3OH Lewis structure October 31, 2023 by Deep The information on this page is fact-checked. CH 3 OH Lewis structure CH 3 OH (methanol) has one carbon atom, four hydrogen atoms, and one oxygen atom. In the CH 3 OH Lewis structure, there are three C — H bonds, one C — O bond, and one O — H bond. And on the oxygen atom, there are two lone pairs.

Spice of Lyfe Methanol Chemical Equation
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.". Lewis dot structure is a pictorial representation of the molecule, it's bonding with other atoms and the arrangement of atoms in the compound. It helps in knowing the number of bonded electrons, lone pairs, and the compound's molecular shape.
Lewis Dot of Methanol (methyl Alcohol) CH 3 OH. Back: 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Methanol (CH 3 OH) is the simplest alcohol which has only one carbon atom. According to the lewis structure of methanol, it has one O-H bond, three C-H bonds and one C-O bond. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. CH 3 OH lewis structure
Ch3Oh Lewis Structure
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of. Drawing the Lewis Structure for CH 3 OH. For the CH 3 OH Lewis structure there are a total of 20 valence electrons available. Remember that Hydrogen (H) only needs 2 valence electrons for a full outer shell. The OH group is attached to the Lewis structure for CH 3 OH as writen in the chemical formula. Put three hydrogens and the OH around the.




