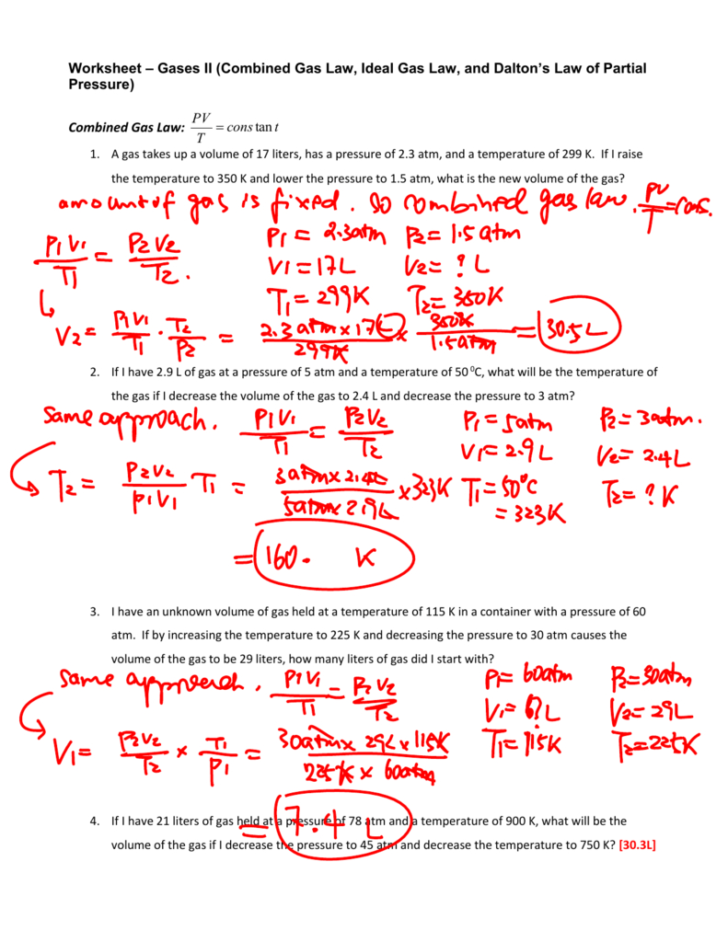
Mixed Gas Laws Worksheet 1) How many moles of gas occupy 98 L at a pressure of 2.8 atmospheres and a temperature of 292 K? 2) If 5.0 moles of O2 and 3.0 moles of N2 are placed in a 30.0 L tank at a temperature of 250 C, what will the pressure of the resulting mixture of gases be? This page titled 2B: Gas Laws II (Worksheet) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Robert Carter. The fundamental relationship PV = nRT can be extended to understand the densities of gases under various conditions and to understand how non-reacting gases behave when mixed together. This and all..
Gas Laws Worksheet 1 Answer Key —
Mixed Gas Laws Worksheet How many moles of gas occupy 98 L at a pressure of 2 atmospheres and a temperature of 292 K? If 5 moles of O 2 and 3 moles of N 2 are placed in a 30 L tank at a temperature of 25 0 C, what will the pressure in atm of the resulting mixture of gases be? Chapter 10 - Lecture Notes. Chapter 9 - Lecture Notes. CHEM EXAM 1 - EXAM #1. Chapter 11,12,13,14,18. Stoichiometry Worksheet #2. Naming Compounds Worksheet. Answer Key mixed gas laws worksheet ke directions: examine cach question and then write the form of the gas law you plan to use to solve cach question. show. 6. 5.36 liters of nitrogen gas are at -25 (C. What would be the volume at 128 (C? 7. At constant temperature, 2 L of a gas at 4 atm of pressure is expanded to 6 L. What is the new pressure? (Do this one conceptually and not algebraically.) Worksheet - Mixed Gas Law Worksheet. 11/19/2014 3:38:00 PM. P = pressure, measured in atmospheres. V = volume, measured in liters. n = amount of gas, measured in moles. T = absolute temperature, measured in kelvins. R = the ideal gas constant, which has a value of 0.0821 L atm/mol K. The ideal gas law was originally developed based on the experimentally observed properties of gases, although it can also.
Worksheet Mixed Gas Laws Worksheet The Gas Laws Worksheet —
MIXED GAS LAWS WORKSHEET 1) How many moles of gas occupy 98 L at a pressure of 2.8 atmospheres and a temperature of 292 K? 2) If 5.0 moles of O2 and 3.0 moles of N2 are placed in a 30.0 L tank at a temperature of 250 C, what will the pressure of the resulting mixture of gases be? Worksheet - Mixed Gas Law Worksheet. Chapter 12: Graham's Law and Dalton's Law of Partial Pressures. 1. How fast would a mole of sulfur dioxide travel if a mole of krypton travels an average of 750 m/s. (Both gases are at 200. o C?) 2. A fluorine gas molecule travels at about 300. m/s at room temperature. Ideal Gas Law. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles ( n) and its temperature, pressure, and volume is given by the relationship. PV = nRT P V = n R T. in which R is the gas constant, with a value of 0.08206 L × atm/K × mol. Worksheet - Mixed Gas Law Worksheet Name: ________________________ SHOW ALL WORK FOR ALL PROBLEMS I. 1.0 atm = 101.3 kPa = 760 mmHg And 0 C = 273 K Change the following units: 359 kPa = _________ atm 10 C = ________ K 6.2 atm = ________ kPa 10K = _______ C For the rest of the problems: First identify each number with P, V, or T.

Combined Gas Law Worksheet Answer Key —
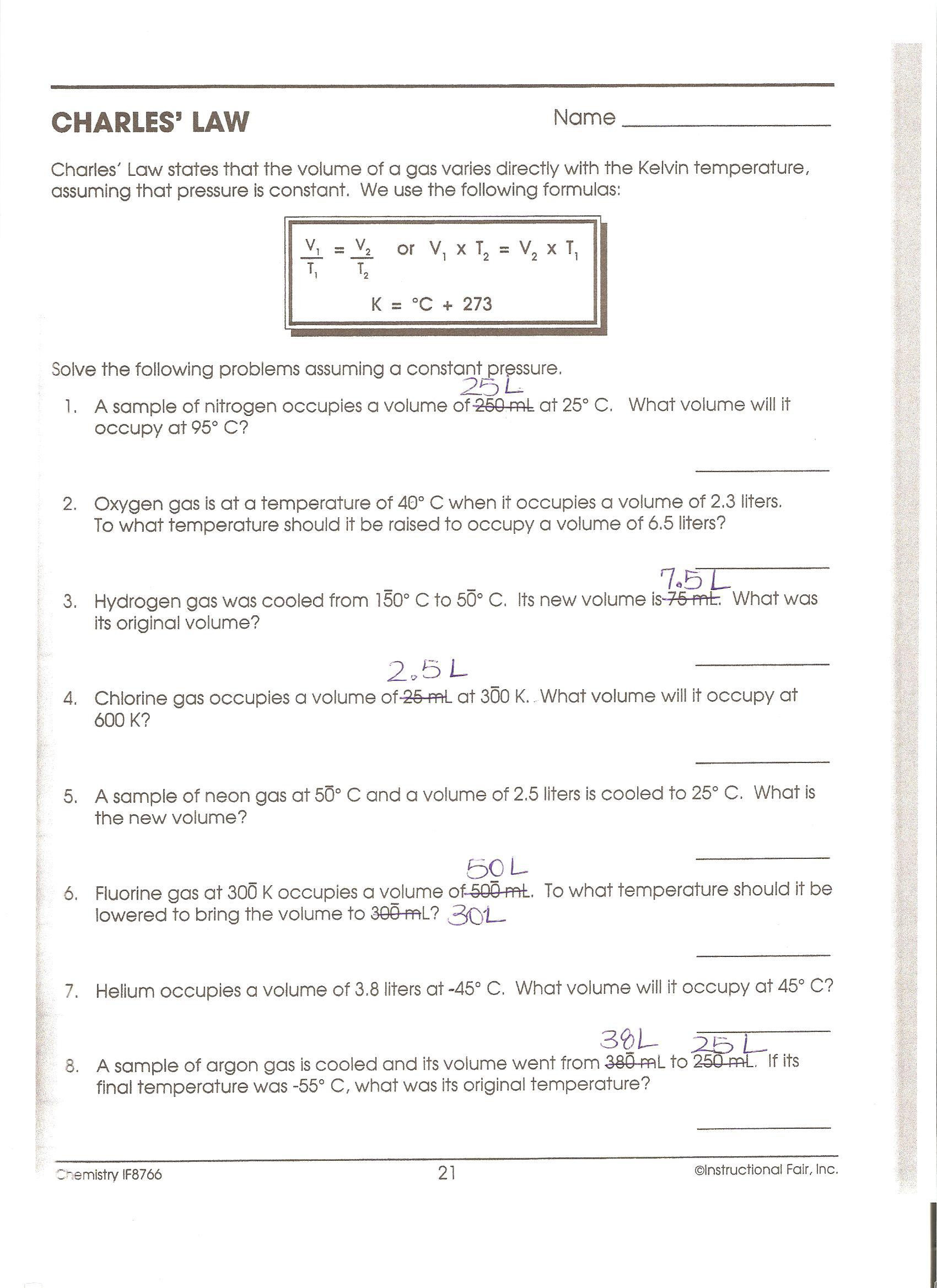
What would the gas pressure in the can be at 520C? (3.27 atm) 2. A sample of hydrogen exerts a pressure of 0.329 atm at 470C. The gas is heated 770C at constant volume. What will its new pressure be? (0.360 atm) 3. A sample of neon gas occupies a volume of 752 mL at 250C. What volume will the gas occupy at standard temperature if the pressure 1. A sample of gas has a pressure of 100.0 torr and 27.0 C. Calculate the pressure if the temperature is changed to 127 C while the volume remains constant. 2. A gas initially at STP is changed to 248 K. Calculate the final pressure of the gas. 3. A gas occupies a volume of 50.0 mL at 27 C and 630 mmHg.
GAS LAW PRACTICE Name: Directions: Choose the correct formula to answer each question, then show your substitution and final answer. Values of "R" are also given above.. MIXED GAS LAW PRACTICE PROBLEMS Author: FCPS Last modified by: mgraniello Created Date: 4/4/2013 12:16:00 PM Company: Fairfax County Public Schools Use your knowledge of the ideal and combined gas laws to solve the following problems. Hint: Figuring out which equation you need to use is the hard part! 1) If four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? 2) If I initially have a gas with a pressure of 84 kPa and a temperature of 350

MIXED GAS LAWS WORKSHEET
Pair-Solo-Teacher :Gas Laws 1. If I have an unknown quantity of gas at a pressure of 0.5 atm, a volume of 25 liters, and a temperature of 300 K, how many moles of gas do I have and what law is this? ( pair) 2. If 6.12 grams of nitrogen gas is held at a pressure of 5.0 atm and in a container with a volume of 50.0 Mixed Gas Laws Worksheet University Miami Dade College Course General Chemistry and Qualitative Analysis (CHM1045) Students shared 153 documents in this course Academic year:2017/2018 Comments or register Recommended for you General Chemistry and Qualitative Analysis (CHM1045) Coursework 85% (34) 19 Week seven Task Sheet Practice materials 100% (7)




