Metal Use the table of ions in Model 1 to answer the following questions: a. In the compound zinc phosphide, what is the charge on the zinc ion? b. In the compound zinc phosphide, what is the charge on the phosphide ion? Explain why a 3 to 2 ratio of ions is necessary for the compound zinc phosphide. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion as illustrated in Figure 5.7.1 5.7. 1 for the compound BaCl 2. The word ion is dropped from both parts. Figure 5.7.1 5.7. 1: Naming BaCl2 B a C l 2.

Naming Compounds
Naming the oxyanions, students don't always see the relationship between the table that helps determine the -ate ions and the one that determines the -ite, per--ate, and hypo--ite ions. Most students put all of the oxyanions with a single negative charge instead of the appropriate charge based on the -ate ion. POGIL ™ Activities for. Naming Ionic Compounds 115 Polyatomic Ions. 123 Naming Molecular Compounds 133 Naming Acids 141 Molecular Geometry. 145 *Page numbers correspond to the Teacher's Edition. iv ™POGIL Activities for High School Chemistry Chemical Reactions and Stoichiometry. Compounds are generally classified as molecular, ionic, or (more rarely) network. Knowing the classification allows us to name the compound correctly and to understand the microscopic organization of it. Describing the fundamental compound unit as either a molecule or a formula unit allows us to determine the mass of that unit. You should be able to write the correct formula for any ionic compound Prerequisites Knowledge of atoms and isotopes Model 1: An atomic look at three compounds The diagrams below represent some ionic compounds at the atomic level. Sodium chloride Chemical formula: NaCl Calcium chloride Chemical formula: CaCl2 POGIL 2005, 2006

Naming Ionic Compounds Worksheet Pogil —
Figure 3.10.1 3.10. 1: A Guide to Naming Simple Ionic Compounds. Follow these steps to name a simple ionic compound. Identify the cation name and the anion name. If the cation can have more than one possible charge, either use the Stock system name of the cation and name of the anion, or use the stem of the cation name and -ic/-ous and the name. How do I recognize that a compound is ionic, and how do I name it? The Model: Compounds Containing Polyatomic Ions (Reference: Section 2.8 in Silberberg 5th ed.) In addition to monatomic ions, there are polyatomic ions. Within a polyatomic ion, each atom is connected to one or more atoms of the same polyatomic ion. The simplest ionic compounds consist of a single type of cation associated with a single type of anion. Nomenclature for these compounds is trivial; the cation is named first, followed by the anion. If the anion is a single element, the suffix ide is added to the root name of the element. When you are constructing names for ionic compounds, you. Figure \(\PageIndex{1}\): A Guide to Naming Simple Ionic Compounds. Follow these steps to name a simple ionic compound. Identify the cation name and the anion name. If the cation can have more than one possible charge, either use the Stock system name of the cation and name of the anion, or use the stem of the cation name and -ic/-ous and the.

Naming Ionic Compounds Worksheet Pogil
Nomenclature, a collection of rules for naming things, is important in science and in many other situations.This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4.The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions. Course: Chemistry library > Unit 1. Lesson 3: Names and formulas of ionic compounds. Naming monatomic ions and ionic compounds. Common polyatomic ions. Polyatomic ions. Naming ionic compound with polyvalent ion. Worked example: Finding the formula of an ionic compound. Predict the charge on monatomic ions. Naming ionic compounds.
a. Identify three elements that form only one cation. b. Identify three elements that form only one anion. c. Identify three elements that form more than one cation. d. In what region of the periodic table are these "multiple ion" elements usually located? 2. Consider the ions of potassium (K) and sulfur (S). Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Image credit: Wikipedia Commons, public domain. Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom.
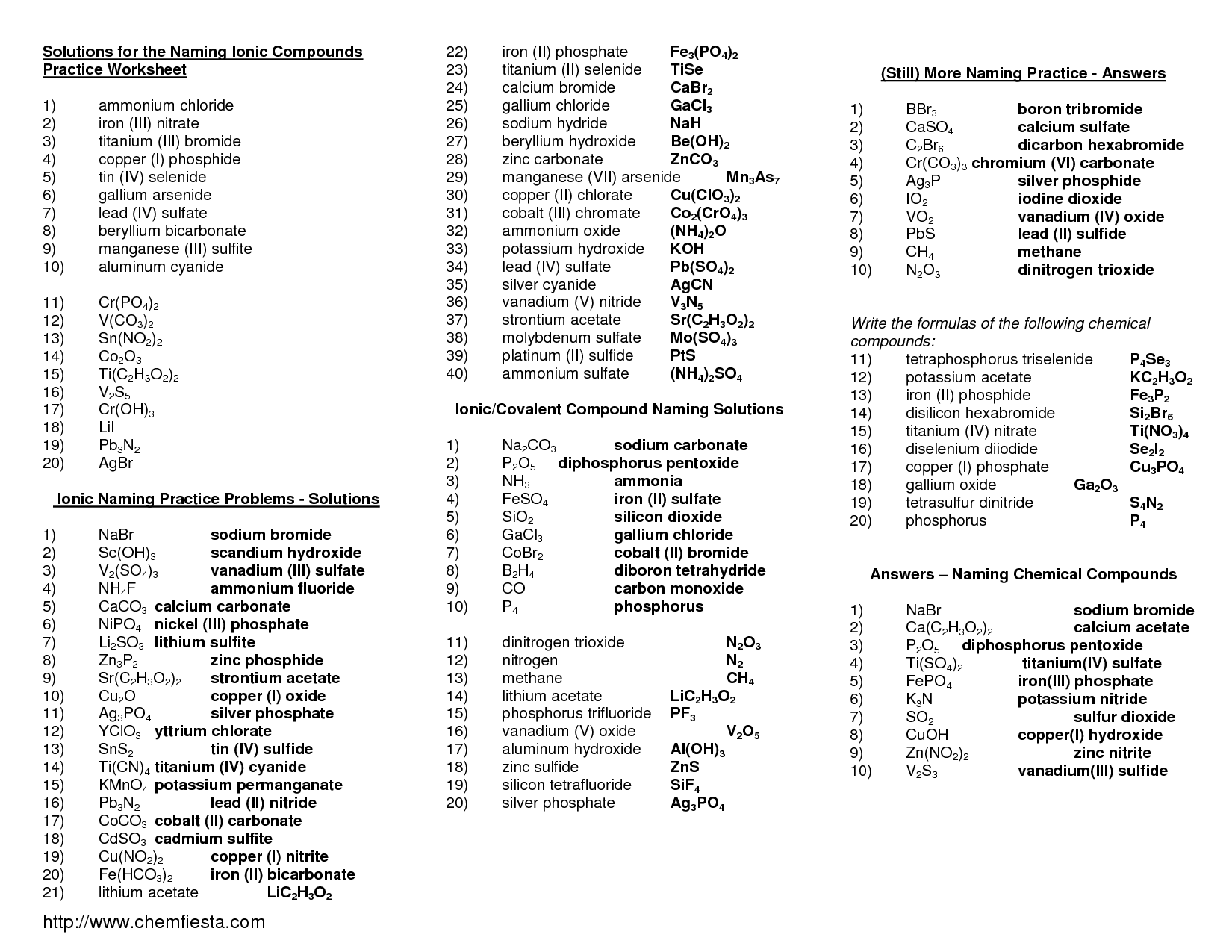
Naming Ionic Compounds Practice Worksheet Resume Worksheets Samples
Terms in this set (3) compound must equal. 0. If a ionic compound forms only one ion then. no change to either name just add ide. If a ionic compound forms multiple ions then. use roman number to specify which ion. Study with Quizlet and memorize flashcards containing terms like compound must equal, If a ionic compound forms only one ion then. Ionic Bonding POGIL Goals: 1) Students will use the periodic table to determine the number of electrons in the valance shell of an atom using their knowledge of electron configurations 2) Students will apply the octet rule in determining how various elements combine chemically using the octet rule




