Atomic Structure (Bohr Model) for Oxygen (O) Wayne Breslyn 725K subscribers Join Subscribe Subscribed 116 Share 22K views 1 year ago In this video we'll look at the atomic structure and Bohr. Now, to draw the Bohr model of the atom for the oxygen we must first understand the various atomic species and their positions inside the atom. They are: • Nucleus: Just like the sun in the solar system, it is the core of the atom. It is composed of two atomic particles i.e. protons and neutrons.

Diagram representation of the element oxygen Vector Image
Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium, it. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is. 1. Find the number of protons, electrons, and neutrons in the Oxygen atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom Single Oxygen Atom Model Cut each of the Styrofoam spheres in half. Discard one half each of the 6-inch and 1-inch wide spheres. Place each of the remaining sphere halves flat-side down on your work surface. Paint the largest sphere one color; blue is traditionally used for oxygen. Paint the large sphere's curved side as well as the flat bottom.

Drawing Atoms Montessori Muddle
The Naked Scientists Periodic Table of Videos Created by video journalist Brady Haran working with chemists at The University of Nottingham. Element Oxygen (O), Group 16, Atomic Number 8, p-block, Mass 15.999. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. How to draw the Bohr-Rutherford Diagram for Oxygen. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. This picture was called the planetary model, since it pictured the atom as a miniature "solar system" with the electrons orbiting the nucleus. Figure 7.3.2 7.3. 2: The emission spectra of sodium and mercury. Sodium and mercury spectra. Many street lights use bulbs that contain sodium or mercury vapor. Due to the very different emission spectra of these elements, they emit light of different colors. The lines in the sodium lamp are broadened by collisions.
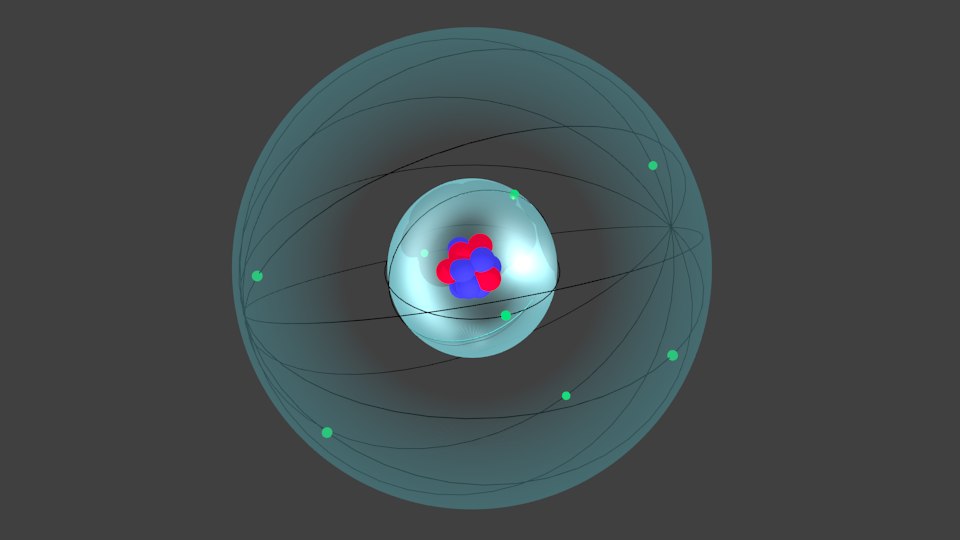
free oxygen atom 3d model
Model of Oxygen Atom - Science Project Atharv 170 subscribers Subscribe Subscribed 102 Share 16K views 8 years ago Project Oxygen - Science Atom Project Fed up of boring old text books and. Lewis structure of O2. The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule.
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). 10.4: The Bohr Model is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Bohr's model suggests each atom has a set of unchangeable energy levels and electrons in the electron cloud of that atom must be in one of those energy levels. Bohr's model suggests that the atomic..

Why does oxygen form the "O"_2^(2) ion? Socratic
Here's how you can draw the Bohr model of oxygen step by step. #1 Write protons, neutrons, and electrons of oxygen atom. #2 Draw nucleus of oxygen atom. #3 Draw 1 st electron shell. #4 Draw 2 nd electron shell. Let's break down each step in detail. This is a model of an Oxygen Atom. Oxygen Atom Structure Model. 3D. Navigation basics; All controls; Orbit around. Left click + drag or One finger drag (touch) Zoom. Double click on model or scroll anywhere or Pinch (touch) Pan. Right click + drag or Two fingers drag (touch).




